We might think of our brain cells as an orderly workforce—filing away memories, tying our shoes, and tidily stringing words together in the proper sequence to order a pizza—but beneath this veneer of harmony, our brains are fraught with infighting and disagreement at every organizational level. Around one third of the neurons produced in a developing human brain are outcompeted for resources and die. Each of our five senses is constantly clamoring for attention. In the cortex, planned movements duke it out for access to our muscles. Much of this competition reflects our physical limitations, such as our inability to move a limb in more than one direction at once, and passes mostly unnoticed. But conflicts between internal drives frequently bubble up into conscious awareness. Consider waking up in the morning and wondering whether to eat breakfast or snag another ten minutes of sleep. Neuroscientists are interested in finding out how the brain monitors internal variables like hunger and sleepiness and how it resolves conflicts between the drives to act on those sensations.
Now, researchers from Professor Kristin Scott’s lab in the Department of Molecular and Cell Biology at UC Berkeley have discovered how a small group of cells in the fruit fly brain resolves the conflict between drives to eat and drink. For humans, with pints of blood flowing through our veins and plenty of excess energy stored as fat, hunger and thirst aren’t usually locked in battle. But, says Nick Jourjine, a graduate student in Scott’s lab, “thirst and hunger are more entangled processes than we’ve previously appreciated.” Flies travel light, with little room to store water and fat, and have a high surface-area-to-volume ratio that makes them prone to dehydration. Too much or too little of either food or water could alter the composition of a fly’s hemolymph—the insect version of blood—and quickly degrade the health of cells throughout its body. So, how do flies’ sand-grain sized brains achieve the right balance?
Any neuron can be tuned to recognize one or more of a wide variety of different stimuli. One neuron might be activated when specialized proteins on its surface detect a particular molecule nearby, while another might integrate electrical signals from distant neurons to perform a computation. Finding a pair of neurons with the same function in two human brains is virtually impossible, but flies’ brains are so much smaller and more stereotyped that scientists can find neurons with the same location, shape, and function in every single fly.
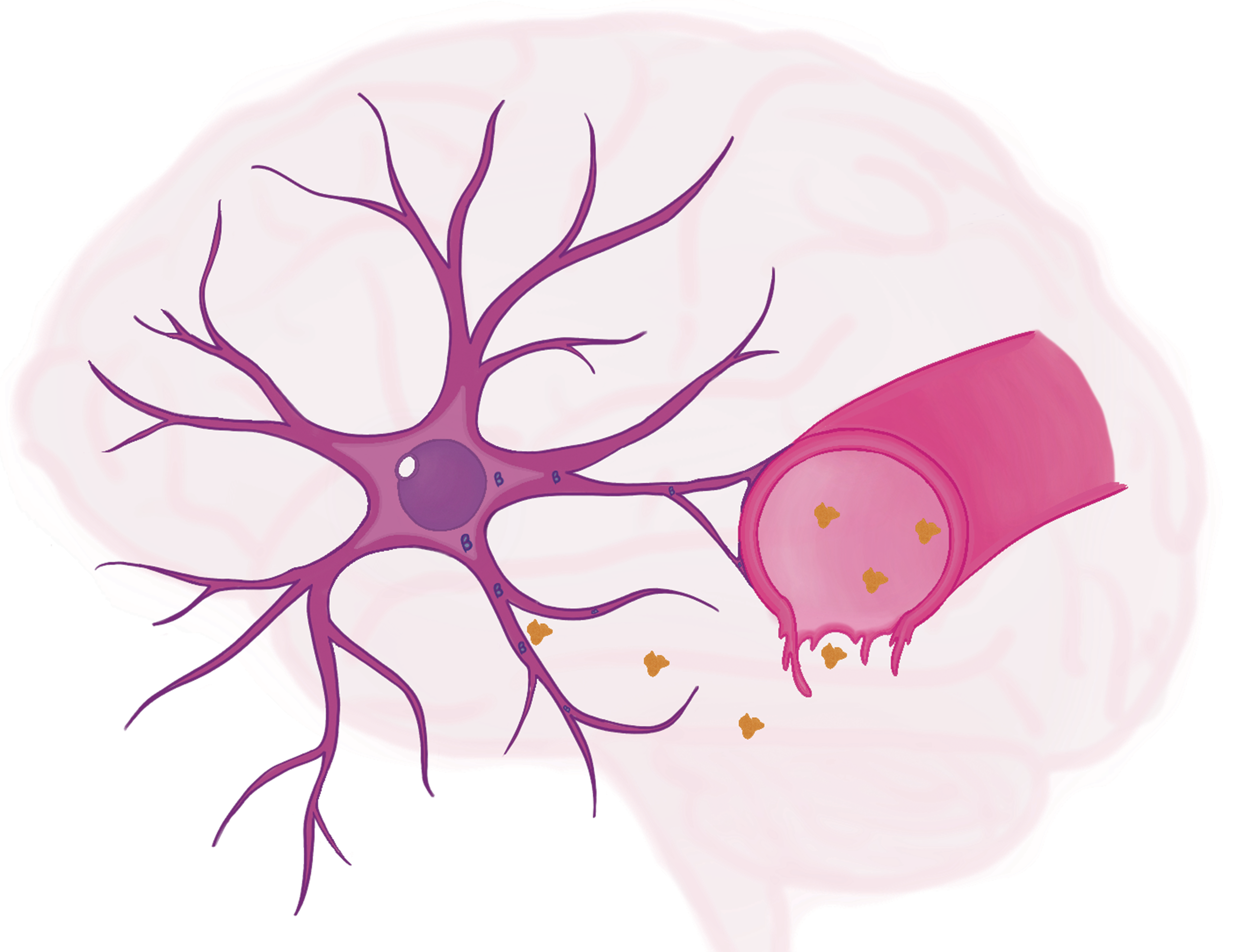
The Scott lab wanted to know which neurons in the fly brain are activated by hunger and thirst and how they detect those signals. They started simply by looking for neurons that, when activated, could make a fly eat, taking advantage of an existing library of genetically modified flies in which small, random subsets of neurons can be activated by heat. One type of fly showed a dramatic result: activating four specific neurons made these flies eat ravenously even when they were full. Next, reasoning that thirst-sensing proteins are crucial for normal water consumption, the researchers used a different genetic tool to systematically disable individual proteins throughout the brain. To the researchers’ surprise, removing a protein called nanchung from the same four neurons that promoted eating caused flies to drink twice as much water as they usually would.
These neurons seemed to exert a powerful effect on eating and drinking, but it remained unclear how they could detect hunger and thirst. Jourjine and colleagues extracted brains from flies whose neurons were engineered to fluoresce brightly whenever they became active and kept the brains alive in a dish of artificial hemolymph. When they bathed the brains in hemolymph containing the hunger-signaling hormone AKH, the four neurons lit up instantly. However, when the water content of the hemolymph was lowered to simulate the inside of a thirsty fly, the neurons’ activity quieted down. Further experiments suggested that these opposite effects on the neurons’ activity are controlled by two types of proteins located on the cells’ surfaces: AKH receptors, which sense hunger and increase the neurons’ activity to promote eating, and nanchung, which senses thirst and decreases the neurons’ activity to promote drinking.
Endowed with these two proteins, a handful of neurons in the fly brain can arbitrate between competing bodily needs. How, then, does the outcome of the nanoscale war between these proteins, waged across the surface of four neurons, shape the behavior of an entire organism? Solving that, the Scott lab hopes, will provide crucial insights into how our brains satisfy our needs.
Zeke Barger is a graduate student in Yang Dan's lab studying the neural basis of sleep.
This article is part of the Spring 2017 issue.
Notice something wrong?
Please report it here.