A middle-aged woman stops in the hospital parking lot. She calmly steps out of her car and takes deliberate, even steps through the hospital’s doors toward the cancer care and treatment unit. This woman has advanced-stage melanoma, and today is her first day of cancer treatment. Instead of being filled with the usual fear of pernicious side effects normally accompanying cancer treatment, she is quite calm. In the coming days, she experiences no hair loss, nausea, or intense fatigue. Instead, our patient experiences the mild flu-like symptoms she expected. While unpleasant, it pales in comparison to the alternative. Following subsequent treatments, her tumors gradually vanish, and she is deemed cancer-free.
What was this wonder cancer drug? As you likely guessed, this was not a mainstream treatment like chemotherapy or radiation leaving a wake of vicious side effects. Instead, our patient defeated late-stage cancer with her body’s own best defense: her immune system.
Nearly every person is born with a powerful immune system able to ward off a wide spectrum of diseases. Despite this ability, the immune system seemingly goes silent against tumors, leaving the body with little to no protection against cancer. Our patient received a treatment that reversed this silence and allowed her immune system to fight back.
By learning more about how the immune system operates, scientists have discovered how to wake it back up to attack a tumor as it would an infection. Such treatment is termed cancer immunotherapy, and it is revolutionizing how we treat this unforgiving disease.
Why is cancer so difficult to treat?
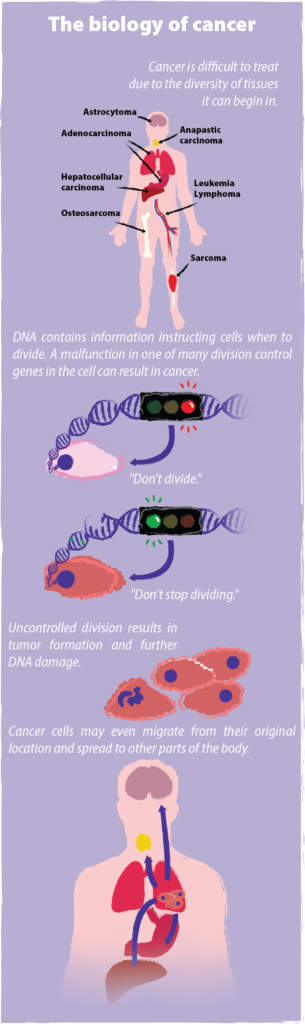
Cancer occurs when normal, healthy cells accumulate mutations in their DNA that cause those cells to replicate rapidly. While healthy cells replicate according to prescribed timetables, cancerous cells break these set timelines and multiply on fast forward. Growing tumors damage surrounding organs and tissue, metastasize, or spread to distant parts of the body, and if left unchecked, cause death. With more than one in three people developing cancer over their lifetime, the disease directly or indirectly touches everyone.
Cancer research and treatment requires immense time and resources not only because the disease is so common and pervasive, but also because it is so difficult to treat. One key problem is that cancer is not a single disease. With over 100 known types of cancer, each takes on a unique identity of its own based on the tissue or organ it originates from. As such, melanoma and brain cancer respond very differently to the same treatment. Even a man and woman with the same tumor type can respond differently to identical treatment. Moreover, as tumors quickly proliferate, they often acquire an increasing number of genetic mutations allowing them to evolve new ways to hide from the immune system or become resistant to cancer-killing drugs. UC Berkeley Professor of Immunology and Pathogenesis David Raulet explains, “Tumors are very subject to selection. You put on a drug, and they often find ways to evade. That turns out to be one of the major problems.” With these and more obstacles standing in the way of combating cancer, it is no surprise that finding effective treatments remains a monumental challenge.
Most mainstream cancer therapies fall into three main categories: chemotherapy, radiation, and surgery. Chemotherapy, or chemo, comes in the form of a drug that selectively kills dividing cells, cancerous or not. Radiation treatment involves bombarding a tumor with x-rays in order to damage and kill cancerous tissue. The drawback to both these treatments is they do not discriminate between healthy cells and cancer cells. Side effects from these treatments arise from the death of healthy cells and range from hair loss and prolonged, major fatigue to a weakened immune system and nerve damage. Raulet says, “There’s essentially zero chance chemo is going to cure you. It gives you time, and time is important, but it is not a cure.” Finally, doctors can attempt to surgically remove a tumor. Surgery can be highly invasive and sometimes involves the removal of an entire non-vital organ or gland. Each of these options comes with clear drawbacks and the risk that some of the cancer will be left behind.
Scientists are constantly searching for better, safer solutions to treat cancer, and research steadily progresses. Cancer immunotherapy has recently leapt into the spotlight as a potential solution with higher precision and fewer physiological costs and is on its way to becoming a leader in cancer treatment.
Hiding from the immune system
In stark contrast to traditional methods, immunotherapy does not kill cancer with a promiscuous drug or exposure to radiation. Instead, it leverages the patient’s own immune system.
The immune system is naturally able to distinguish between healthy and unhealthy cells and is composed of many types of specialized cells to do so. One class of cells, known as T cells, rely on reading short protein fragments, or peptides, displayed on the surface of other cells via a molecule called Major Histocompatibility Complex, or MHC. These peptide fragments are derived from the many proteins found within a particular cell. T cells will not turn on the immune system when they encounter MHCs loaded with peptides from a healthy cell. Such peptides are referred to as self-peptides since they originate from the cell itself. Alternatively, if the cell is infected with virus or bacteria, a few MHCs will now carry fragments from foreign proteins. These foreign peptides are known as antigens. T cells can discriminate between self-peptides and antigens with exquisite accuracy. When a T cell identifies an antigen, it sounds the alarm to the rest of the immune system to unleash an attack on all unhealthy cells carrying that antigen.
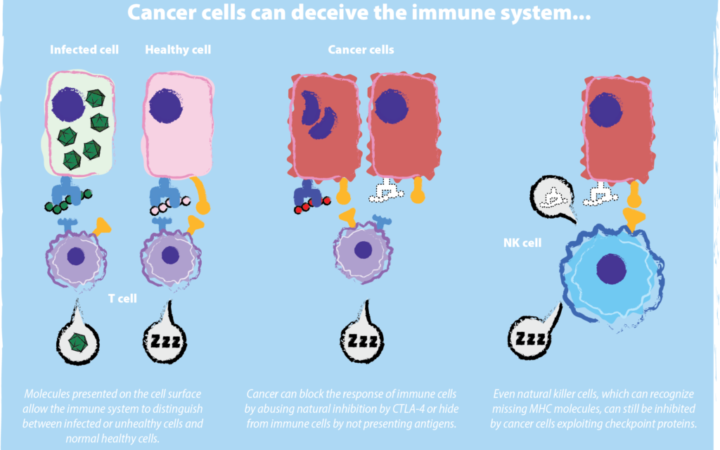
Importantly, mutated self-proteins, like tumor-derived proteins, may also act as antigens and have the potential to induce an anti-tumor T cell response. However, tumor cells have a deep bag of tricks to hide themselves from the immune system. For one, they may appear to look healthy on the outside by lacking a recognizable antigen or MHC. They can also shut down tumor-specific immune responses by forcing T cells and other immune cells into an unresponsive, sleep-like state. By these and other means, tumors evade immune surveillance.
What is cancer immunotherapy?
The goal of cancer immunotherapy is to draw back a tumor’s cloak by turning a patient’s immune system back on and enabling it to clear the cancer as it would the flu. Berkeley Professor of Immunology and Pathogenesis Russell Vance says, “The immune system has a really remarkable ability to hunt down specific cells that are different in some way from normal cells and eliminate them. It’s surgical in that way, and that really lends itself to [killing] cancer... You are taking advantage of the millions of years of evolution and applying it to a different target.”
Traditional therapies are directed at tumors and by nature, apply selective pressure leading to evolved tumor resistance. Tumor cells that are susceptible to the treatment die, but cells that are not continue to grow. On the other hand, immunotherapy targets and bolsters the immune system. By treating the immune system, cancer is far less likely to develop resistance. Vance elaborates, “The hope is that we can actually develop durable cures for [cancer].”
Another possible benefit of targeting the immune system is having a treatment that is cancer-type agnostic, meaning that, in theory, it could treat any type of cancer, from lymphoma to bone cancer.
Early criticism
Though the potential for immune-based cancer treatment seems obvious, the road to acceptance was long and rocky. At the turn of the twentieth century, Dr. William Coley first played with the idea of fighting cancer with the immune system. However, his results were unrefined, mixed, and met with great criticism. At the time, extremely little was known about the immune system and how it operated; T cells had not yet been discovered. Coley certainly did not know how his therapy, Coley’s Toxin, occasionally worked, and for most of the twentieth century, the general scientific body considered cancer immunotherapy to be junk science.
To make matters worse for the field, a cancer immunotherapy hype boomed then busted in the 1980s as a putative therapy failed to deliver on promised expectations, leading to even greater skepticism and criticism.
The existence of modern cancer immunotherapy is largely thanks to Dr. James P. Allison, an immunologist who built his career and reputation in this field at the UC Berkeley and whose tenacity helped ensure its permanence in the future of cancer treatment.
Opening the floodgates
By the time he arrived at Berkeley in 1985 as a professor of immunology and director of the Cancer Research Laboratory, Allison had already gained some prominence as the first person to find the T cell receptor, the molecule on the T cell’s surface that identifies antigens. His lab studied another protein presented on the T cell surface, CTLA-4. They discovered that CTLA-4 acted analogously to the brake pedal on a car. If CTLA-4 is activated, the T cell halts its attack. These brakes are referred to as checkpoints, and they are a crucial protective mechanism to prevent T cells from killing healthy cells.
Allison speculated that tumors may develop a way to exploit the T cell’s natural brake system to keep the brakes permanently on, thereby evading the immune system. If true, one could feasibly stop the brake pedal, from being pressed and turn the immune cells back on to attack the cancer. He was right. Allison and his lab decided to use an antibody, a Y-shaped molecule that tightly binds to a given target, to physically block cancer from pressing the brake pedal and in 1996, showed they could clear tumors from mice.
Despite this success, Allison’s fight was far from over. Skeptical pharmaceutical companies were completely unwilling to take the risk and invest to take the therapy to clinical trials. Raulet, who was a colleague of Allison during his time at Berkeley, recalls that the cancer treatment industry “was not paying attention to the immune system.” Vance believes that Allison’s persistent pressure against drug companies was pivotal for immunotherapy to succeed, adding that Allison “really strongly believed in this idea, and he pushed on drug companies to follow up on it. Without that, the drug would have been dropped.” Inspired by a personal family history with cancer, Allison maintained unwavering conviction that his treatment could work. After several companies refused, Allison went to Bristol-Myers Squibb for a second time, and they agreed to give his therapy a try.
Finally, after fifteen tenacious years, James Allison defied the odds with a landmark victory that permanently sealed cancer immunotherapy’s place in modern medicine when his antibody-based therapy became FDA approved in 2011 as ipilimumab, trade name Yervoy, to treat late-stage melanoma. Therapies such as Allison’s became known as “checkpoint blockade” and his antibody— which inhibits activation of checkpoint proteins, such as CTLA-4—as a “checkpoint inhibitor”. Several checkpoint inhibitors are now available with many more immunotherapy treatments currently in clinical trials.
Allison’s triumph is touted as having “revolutionized cancer treatment” and eventually led to him receiving the 2018 Nobel Prize in Physiology or Medicine, which he shares with Dr. Tasuku Honjo, who performed similar work on a different checkpoint molecule, PD-1. Their efforts opened the floodgates for a worldwide race to develop new immune-targeted therapies, much taking place in the Bay Area. Cancer immunotherapy is now a well-accepted, blossoming field. Vance explains that “what was revolutionary about Jim’s works was that he showed it could work. That has been hugely influential and transformed how a lot of companies are going about looking for cancer therapies.”
Now, unprecedented success stories of cancer patients are periodically reported. For example, a woman in 2018 with golf ball-sized, metastatic lesions in her liver and chest was completely cured after a single immunotherapy treatment.
As of April 2019, the full collection of immunotherapies has gained approval to treat over 20 different cancers with over a hundred new treatments currently in clinical trials. The scope of immunotherapy approaches is also ever expanding, from developing tumor vaccines to genetically engineering immune cells with the ability to recognize tumor-specific antigen. Cancer immunotherapy treatments have already saved tens of thousands of lives leading it to be coined by some as “The Beginning of the End” of cancer.
Current immunotherapy research at Berkeley
Allison did not begin his work on CTLA-4 with the intention of creating a cancer treatment. He simply wanted to do basic research to understand how the immune system worked. He also had the perspective and vision to recognize the potential application and breakthrough of his research. Allison often says that if he had been studying how to treat cancer, he would have never discovered CTLA-4 treatment. His story underscores two important lessons: the critical importance of basic, fundamental research and the need for scientists across diverse disciplines to address common issues.
UC Berkeley now houses the Immunotherapeutics and Vaccine Research Initiative (IVRI) with the mission of targeting the immune system as a therapy against disease. Professor Raulet is the faculty director of the IVRI and actively studies cancer immunotherapy in his campus laboratory. Raulet’s lab focuses on a different type of immune cell known as a natural killer (NK) cell.
One challenge with using T cells to identify and attack tumors is that they require antigen, and a major source of antigen is mutation. “The success of the checkpoint therapies is correlated with the number of mutations,” Raulet explains, “[and] many tumors just don’t have any antigens. This is a big problem in the immunotherapy era.” The antigen problem is where NK cells offer a unique advantage. They do not need antigen. Therefore, NK cells have the potential to combat tumors that are less accessible to T cells.
NK cells have two main modes of recognizing cancer and initiating a reaction against it. First, NK cells recognize MHC molecules regardless of whether they contain antigen. All healthy cells have MHC, so if a cell lacks MHC, NK cells will notice, turn on, and kill that cell. Several types of tumors become MHC-deficient as a ploy to hide from T cells because if a tumor lacks any MHC, it cannot display antigens. However, in this scenario, NK cells can see and attack this subset of tumors that T cells cannot. NK cells are also activated by cellular stress. Stress pathways include hyper-proliferation and DNA damage—two processes that can occur in cancerous cells. These pathways essentially wave a red flag to NK cells that the stressed cell is defective and needs to be terminated, and since “cancer cells are very stressed out,” as Raulet comments, they give off this signal to NK cells.
A natural question is if NK cells have such a strong potential, why do they not attack cancer as soon as it emerges? Raulet explains, “It’s the same problem T cells have. You frequently find NK cells inside tumors, and they are shut down, they’re exhausted, they’re depleted, they’re desensitized. Our job is to wake them up, and that’s what we spend a lot of time trying to do.”
While one way to turn on NK cells is to use the classical checkpoint inhibitors, the Raulet lab is also finding promise in another immunological tool, the cGAS-STING pathway. This particular pathway is activated when DNA is found outside of the cell nucleus, which sometimes arises as a result of genetic mutations that contribute to cancer. When the protein cGAS finds DNA out of the nucleus, it helps make a molecule called cyclic dinucleotide. Cyclic dinucleotide switches on the protein STING, which ultimately activates an immune response.
Raulet’s team found that injecting cyclic dinucleotides directly into a mouse tumor activates cGAS-STING, and the NK cells wake up. Their research further shows that the therapy effectively combats tumors beyond the initial tumor site injected with cyclic dinucleotide. “You inject right into the tumor, so it is a local therapy. The problem with local therapy is that you don’t want to just treat the local tumor. You want to treat metastasis. We are seeing these distant site effects where you treat one tumor, and a second one in the mouse is affected.” By combining this treatment with other immune activators, the immune system can respond against cancer even more effectively.
With each cancer being its own distinct disease, NK cells offer an additional tool to the cancer immunotherapy collection, especially in tumors where T cell-based therapies are ineffective. In addition to having a strong response on their own, NK cells also play a special role in waking up and recruiting T cells. Raulet explains, “There are going to be scenarios where the NK response is going to be helpful or additive [to a T cell response], but there will be other scenarios where T cells can’t do it,” and NK cell by themselves offer a better option, personalized based on patients’ specific tumor type.
Current obstacles
With all its advantages, current immunotherapy treatments have limitations, flaws, and some side effects. Unfortunately, immune-based therapy does not work for all patients. Allison’s revolutionary checkpoint inhibitor, for example, is only effective in approximately 20 percent of patients. So, while the number of tumors being treated is steadily growing, immunotherapy has a ways to go before it is in reach to all cancer patients.
Some of the ongoing challenges involve creating treatments that can access different types of tumors. Immunotherapy is most effective at combating cancers such as melanoma and other non-solid tumors; however, solid tumors, such as cancerous masses of the breast or colon, remain a formidable challenge. Inside solid tumors, the chemical environment is especially harsh and suppresses immune cells, making it even more challenging to keep them awake and actively destroying the tumors.
The paucity or lack of antigen for the immune system to recognize continues to be a major challenge for T cell-based therapies.
Furthermore, there are health risks to hijacking the immune system. One major concern is hyperactivating the immune system and causing a reaction similar to autoimmunity, where immune cells destroy healthy cells. This hyperactivation, while fortunately uncommon in most immunotherapy patients, can result in severe inflammation in organs such as the kidneys, liver, lungs, glands, or brain. More likely side effects manifest as flu-like symptoms.
In the face of limitations and safety concerns, it is imperative to continue supporting fundament research to yield solutions to many of these challenges.
A promising future
As researchers quickly move to close the gaps, they are discovering creative new ways to safely coerce immune cells to find and attack cancer. For instance, scientists and doctors recently found that combining checkpoint with chemo or radiation therapy greatly increases the efficacy either can achieve alone. “Immunotherapy and these traditional methods show evidence that they can synergize,” explains Vance. In fact, former United States President Jimmy Carter was cured of melanoma, which had spread to his liver and brain, by such a method. Vance sees immunotherapy as a part of the arsenal to treat cancer. “Each cancer is its own problem. I think tumor immunotherapy will be part of the solution in combination with other approaches.”
Raulet has great optimism in the future of cancer immunotherapy as well. He says that checkpoint therapy “is only a small fraction of what we can do. I certainly hope we can get rid of chemo and radiation.” While that potential may be in the distant future, the realization of its possibilities is approaching ever closer.
Cancer is a devastating disease that touches every person’s life in one way or another, and treating it requires more attention than ever. Despite obstacles, scientists are confident that these are just the first chapters of immunotherapy, and the best is yet to come. The opportunity to offer patients a low-risk, personalized, and lifelong-lasting cure without brutal side effects is a tantalizing prospect. Even a partial realization of this vision will save countless lives. Thousands are already in remission thanks to immune-based therapy, and at the current rate of progress, we may likely be one of those cancer patients cured by cancer immunotherapy.
Mark O'Dair is a graduate student in chemistry
Design: Santiago Yori
This article is part of the Fall 2019 issue.