Making carbon pull its weight
How scientists are reimagining our carbon future

The sustainability problem you haven’t considered
You are no longer shocked to hear about the latest super storm or that we have once again experienced one of the hottest years on record. You know that we have already seen the initial effects of excess carbon dioxide (CO2) in the atmosphere and that moving away from carbon-based fuels as soon as possible is vital to avoid the most devastating effects of climate change. But when thinking critically about decarbonizing the economy, you soon realize that in addition to the myriad social and political obstacles, there are unavoidable logistical and practical questions that make completely ridding ourselves of carbon a near impossibility. For instance, plastics are made of chains of carbon atoms, so they cannot be produced without a carbon-based starting material. Can we eliminate all plastics in all sectors of daily life and commerce? It is unlikely any time soon. Electric vehicles are becoming more popular every year, but can we realistically make an electrically powered cargo ship? The size and weight of the battery that would be required makes one difficult to imagine. As we undergo the transition from using coal, natural gas, and petroleum toward using wind, solar, and hydroelectric for electricity production, how can we make sure that the new grid is as efficient as the old one? Because these renewable energy sources produce power on intermittent and sometimes unpredictable schedules, they require a durable and cheap way to store a large amount of excess energy, so it can be utilized later. Currently, our options for such energy storage technologies are limited.
Despite the anxiety that this discussion surely provokes, this article is not a therapeutic rant about the impossibility of our situation. It is a recognition that electric power from renewable sources is key in the climate crisis, but it cannot be our only strategy for achieving net zero CO2 emissions. Some of the top researchers in climate-related fields at several of the US national labs, including Lawrence Berkeley National Laboratory (LBL), authored a recent roadmap focused on “closing the carbon cycle” published in Nature Reviews Chemistry. The authors describe the “hard-to-electrify” sectors of the economy, which include both heavy-duty transportation and plastics production, accounting for roughly 20% of current greenhouse gas emissions. In these areas, renewably generated electricity will not be enough to reach net-zero CO2 emissions in the near future. The authors argue that it will therefore be necessary to employ non-carbon-based fuels, like hydrogen (H2) and ammonia (NH3), as well as a strategy of sustainably “keeping carbon in play” through sequestration and long-term reuse within durable and recyclable goods. Because of the dire need for technological advancement to help us meet our climate goals, the authors also advocate for a paradigmatic shift in the way that much research is performed in materials science, chemistry, and engineering. They define “use-driven” science as a program of research that focuses on industrial applications from the outset and performs careful analyses to identify any economic or technical issues that may arise when scaling up to a commercial product. They contend that such a mindset will be key to achieve net-zero emissions on the timeline that the planet requires.
Making carbon renewable
To close the carbon cycle, you must use the vast and continually growing carbon feedstocks or reservoirs of carbon-based waste products. The authors of the roadmap identify the major carbon feedstocks as agricultural byproducts (or biomass), personal food waste, plastics, and the carbon dioxide and methane that is already in the atmosphere. If these sources of carbon can be sequestered as useful materials and continually reused over decades or even centuries, they are prevented from being converted into atmospheric CO2. The plastics industry is in a tremendous position to assist with carbon sequestration, if it uses the right materials and puts in place the right manufacturing procedures.
Right now, a depressingly low figure of around six percent of plastic waste in the United States is successfully recycled. This leads not only to the saturation of our landfills and the destruction of ocean habitats, but also to the production of even more plastics—only adding to the greenhouse gas problem. The social component of insufficient recycling cannot be ignored, but lower costs and greater efficacy through materials research may lead to an increase in involvement from both corporations and individuals. Dr. Brett Helms, a materials scientist at LBL and an author of the roadmap, has worked on the development of a “circular plastics economy,” in which nearly all plastics are efficiently recycled with minimal energy input. Recent work from Dr. Helms and colleagues paves the way for using carbon feedstocks, such as agricultural waste, as the building blocks for renewable plastics.
All plastics are composed of polymers—long chains of the same chemical group, or monomer. To chemically recycle and reuse a plastic, you need to break down the polymers into monomers and then reassemble them. This is difficult because decomposability and the durability for which plastics are valued form opposing objectives. However, in 2019 Dr. Helms and his coworkers showed that a particular class of polymer, polydiketoenamines (PDKs), is especially amenable to recycling while also retaining the typical characteristics of a plastic. Dr. Helms cites the various versions of PDKs as having, overall, “the lowest cost, lowest intensity recycling process of any plastic that exists on the market.” PDKs stand out among other types of polymers because the bonds that connect the monomers are “incredibly customizable and programmable.” This means it is easy to design a PDK-based material in which the bonds are strong and durable under most conditions but can readily break during a specific chemical recycling process.
The unique chemical properties of PDKs make them great candidates for building a sustainable plastics economy. However, because recycling can never be performed with 100% efficiency, a truly sustainable plastic should be produced as much as possible from carbon-based waste products rather than manufactured precursors. In a 2023 article published in Nature Sustainability, Dr. Helms, first author Jeremy Demarteau, and colleagues took a major step in that direction by creating a PDK through a biological synthetic process that could be fueled by agricultural waste products such as corn stover. In a typical synthesis of a PDK polymer, a petrochemical called dimedone is used to create the individual monomers. In the “biosynthesis,” a similar compound called triacetic acid lactone (TAL) is used instead. The “bio” piece comes into play because TAL is a natural byproduct of a metabolic process in certain strains of the common bacteria Escherichia coli. Thus, in the proposed manufacturing process, glucose is fed to a batch of E. coli, which naturally produces TAL. The TAL is then easily collected and used to create the PDK monomers. The glucose starting material could be obtained from corn stover, thereby sequestering carbon from an existing feedstock while lowering the cost of the manufacturing process overall.
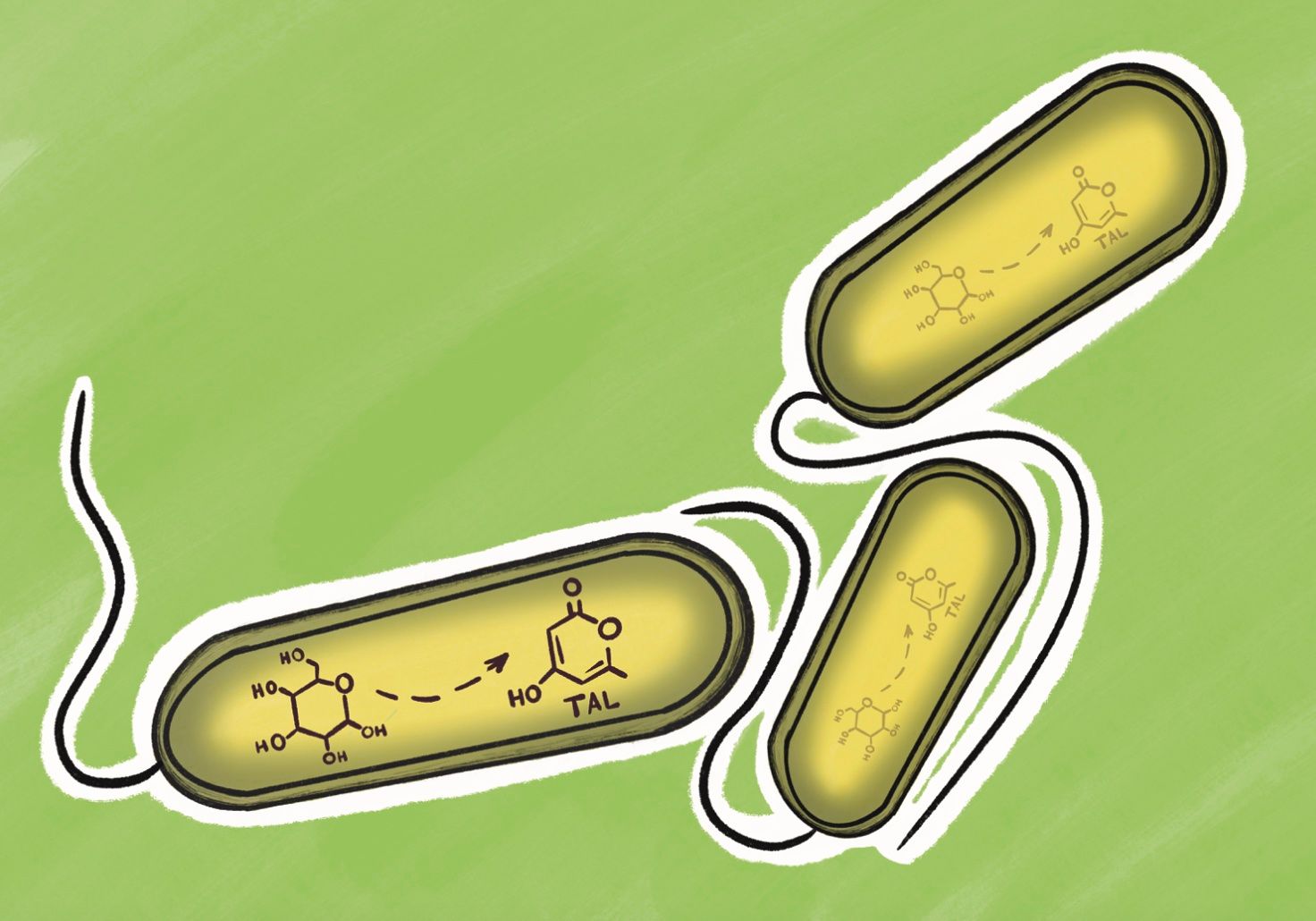
Advances like these are crucial to increasing the appeal of renewable plastics, so that the industry will shift towards a more sustainable model. But, as Dr. Helms puts it, “even if replacing a petrochemical with a biologically produced compound is the virtuous choice, few people are going to do it if the biomolecule is more expensive.” Enter “use-driven” science. Dr. Helms and colleagues carried out a detailed systems analysis of the TAL production through biological methods, calculating the cost and greenhouse gas emissions of each step in a real-world manufacturing process. Their analysis suggests that, in an optimized production scheme, TAL could be produced at a significantly lower cost and with significantly less lifecycle greenhouse gas emissions than the petrochemical that it would replace. Of course, such theoretical systems analyses can never predict every possible challenge in large-scale production, but Dr. Helms nonetheless emphasized their utility in our conversation. He believes that a complete lifecycle analysis, even very early on in the development of a new technology, “gives you a current status of the technology and identifies where the pain points are,” adding that “you can use that knowledge to direct future efforts and eliminate the sources contributing to a greenhouse gas profile that are easiest to address.”
Replacing carbon fuels
Using carbon-based materials in a sustainable way without ever forming CO2 is only feasible for cases where you can carefully control the chemical reactions that the carbon-based compound undergoes, such as during chemical recycling. However, burning carbon is a different story. When you combust compounds that contain carbon atoms, you typically end up with CO2 because that is the point past which no further chemical reactions will occur. CO2 is one of the most stable forms a carbon atom can take. It is hard to avoid producing CO2 and equally challenging to capture all the CO2 produced. However, CO2 production can be completely avoided simply by using a fuel source that has no carbon in it. The investigation of one such fuel, H2, as a method of energy storage is an active priority for the US Department of Energy (DOE) as one of their Earthshot projects. The DOE’s aim within the next decade is to be able to produce one kilogram of H2 for one dollar without any greenhouse gas emissions. However, both this goal and the incorporation of H2 into the energy economy come with significant challenges.
For H2 to be a useful substitute for carbon-based fuels, one must not only produce it at scale via a carbon-free method, but also transport it efficiently, store it safely, and access it easily. While all four of these steps remain difficult with our current technologies and infrastructure, some researchers remain optimistic that H2 will have a role to play in near-term decarbonization. Dr. Francesca Toma is a visiting professor at LBL and the director of the Institute of Functional Materials for Sustainability at Helmholtz-Zentrum Hereon. She has worked on the production of H2 by splitting water with sunlight, one of the most promising carbon-free methods of production. In this procedure, a solar cell is surrounded by a water and salt solution. Sunlight hits the solar cell, causing charge separation (where electrons move to one side). Once the charge is separated, a catalyst is used to initiate the water-splitting reaction at the interface between the metal and the water, generating H2. The major problem with applying this method in practice is that you need the chemical reaction to proceed quickly while not degrading the material of the solar cell over time. You would expect that, due to this degradation, the efficiency of H2 production via water-splitting will decrease steadily as any device is used. However, in 2021, Dr. Toma’s group elucidated the mechanism by which a gallium nitride (GaN) coating of a silicon solar cell unexpectedly increases the efficiency of H2 production over time. Using both theoretical methods and direct measurements, Dr. Toma, first author Guosong Zeng, and colleagues demonstrated that, as the device operated, oxygen atoms incorporated themselves into the GaN coating, which produced a new outer layer, both protecting the silicon and creating more active sites at which the water-splitting reaction could occur. Understanding how and why this unexpected, concomitant increase in stability and performance occurred was a major step toward commercial production of H2 via this technique.
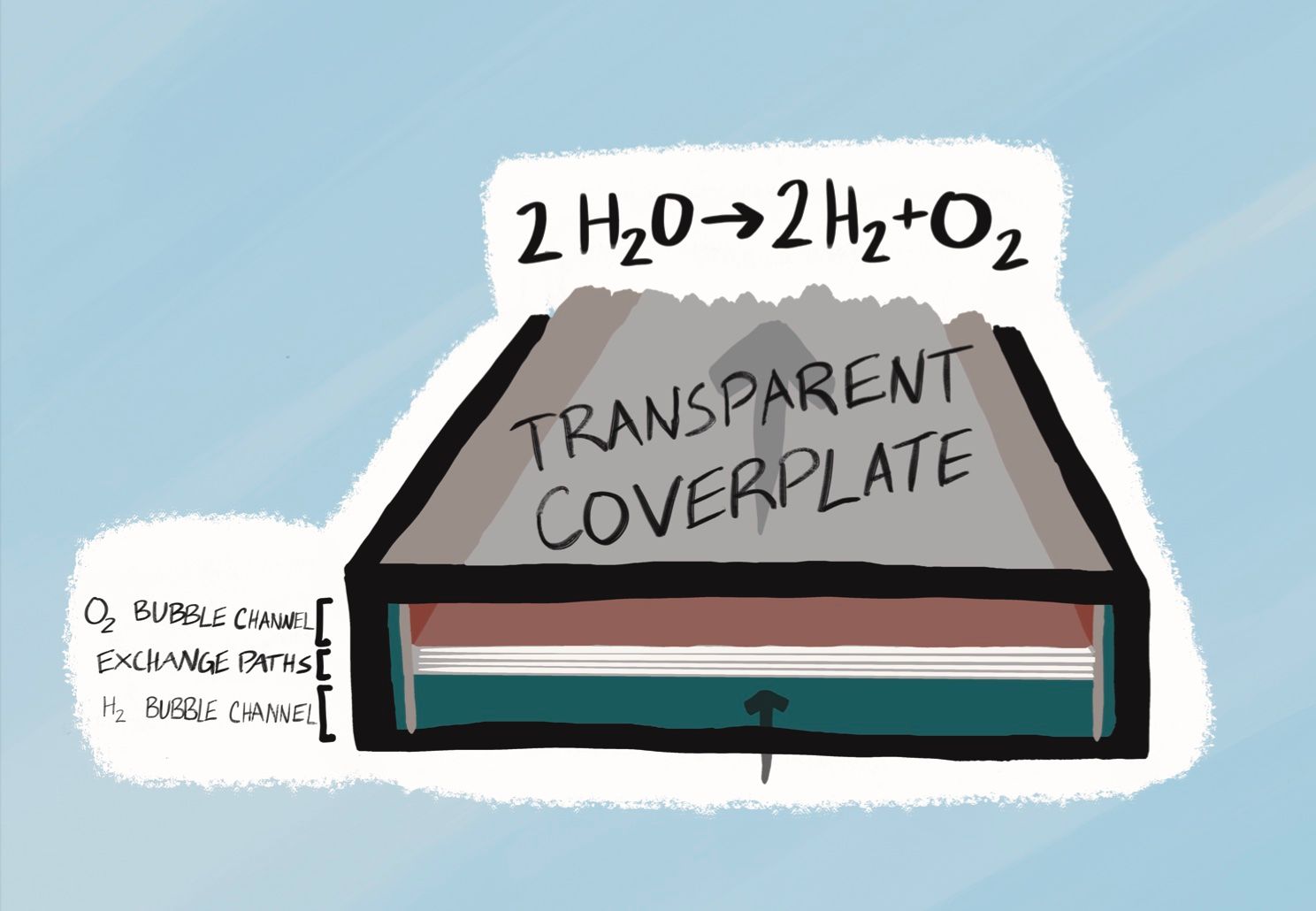
Water-splitting could one day be a key piece in a completely renewable grid. Solar power generation needs the sun, which means it only produces power on an intermittent and often unpredictable schedule. Therefore, a purely solar grid requires an efficient and stable way to store excess power, so it can be used when the sun is no longer shining. Chemical batteries are a natural choice for energy storage, but hydrogen might be an even better option. Dr. Toma believes that the higher energy density of hydrogen as compared to batteries gives it a “huge advantage,” but also emphasizes that “we as society need to become familiar with the idea that there is not a ‘one-size-fits-all’ solution. Hydrogen will play a role, so will batteries.”
Nevertheless, the ability to store H2 safely and stably for long durations becomes vitally important if it is going to be integrated into the electric grid. Additionally, if you wanted to use H2 directly for fueling something like a cargo ship, it would be necessary to transport it long distances. Storing and transporting H2 as a gas is problematic due to safety concerns and practical challenges, such as the volume of the containers that would be required, but there are other options. In the roadmap, the authors highlight the promise of liquid organic hydrogen carriers (LOHCs). LOHCs are both a potential solution to the problem of transportation and storage and another way to use carbon feedstocks. In an economy based around LOHCs, H2 would be produced through a carbon-free method like water-splitting. Then, a chemical reaction would be performed with some organic compounds to create a hydrogen-containing liquid (LOHC), which could be transported using existing pipelines and infrastructure. Finally, the reverse chemical reaction would be performed to access the hydrogen when and where it is needed. If the organic compounds used for this procedure are compounds found in carbon feedstocks such as agricultural waste and biomass, the LOHC can both sequester carbon while enabling carbon-free energy production. There are even hopes that atmospheric CO2 itself could be used to form an LOHC, which would lead to energy production with not just net-zero but net-negative carbon emissions. The problem is finding the right compounds and the right synthetic procedure to make the LOHCs. In general, if a chemical reaction occurs reliably and quickly in one direction, it must proceed slowly in the reverse direction. The key to making LOHCs work is to find specific organic compounds, catalysts, and conditions such that the reactions both to store and recover H2 do not require significant energy input. The authors argue that looking for such reactions should be a priority in H2 research over the next several years.
Circular plastics and sustainable H2 production are just two of many areas where we need rapid technological improvements to help fight climate change. In almost all of these areas, there is active research in national labs and universities and concurrent efforts in industry, but often academia and industry are not entirely in step with each another. Discoveries and advancements achieved in academia point toward applications down the line but require significant reworking on the industrial level to achieve commercial viability, thus creating a lengthy gap between the conception of useful technology and its implementation. This process has been implemented for decades, but the authors of the roadmap contend that, when it comes specifically to the climate, we might not have the time to wait for this slow trickling of ideas from the lab bench down to the factory. They argue it is necessary to accelerate this process through scientific discovery that is intentionally and directly aimed at useful technology on a large scale.
Efforts like those of Dr. Helms and Dr. Demarteau are particularly important in sustainability-focused research because they explicitly consider how their new manufacturing process for PDKs might scale to the industrial level. A scientist at a lab bench cannot predict all the obstacles that might be encountered in a commercial realization of their research, but they can keep in mind relevant practical concerns as they consider offshoots to current projects, and they can look for solutions to known logistical problems within fundamental science. Furthermore, the construction of a research program itself can be informed by “use-driven” goals. Dr. Helms suggests that, in technologically focused research, a principal investigator should ask themselves, “Through what means of stakeholder engagement have you informed your perspective, and is that perspective enlightening with regard to the most important factors for solving the problem?” Such perspectives can be formed in part through direct engagement between scientists and industrial partners. For instance, Dr. Toma participated in a panel discussion and review article on how the materials science community can work in support of industrial efforts for the creation of a sustainable H2 economy. She argues that this type of interaction with industry “brings you back to reality,” suggesting that scientists too often focus on the aspects of a new problem that are like the work they have done previously. Staying within one’s expertise can lead to productive research and deep understanding, but by interacting with industry, one can know when it will be advantageous to move out of their comfort zone to apply their expertise in a way that will have the most societal impact.
Academic researchers and those in industry are increasingly recognizing the value of collaborative efforts, particularly when it comes to addressing problems that, like climate change, require solutions on a global scale. Dr. Toma hopes that publications like the roadmap will “inspire a younger generation of scientists to realize that understanding fundamental physics is important, but then we need to use fundamental physics and chemistry to solve our problems.” And, at the end of our conversation, Dr. Helms argued that a “use-driven” mindset even makes science more interesting itself, claiming that “it is the interplay between the knowledge-building and the use-cases that makes for a rich area of research.” He closed out by reminding me that, “no matter how much we scientists get excited by the nitty-gritty details, at the end of the day, you need to demonstrate value.”
This article is part of the Fall 2024 issue.



