Extending quantum equations to new elements
There's nothing wrong with a little mathematical approximation
What makes up matter—all the physical substances around us—is a question that has confounded scientists and philosophers for millennia. While Democritus, an ancient Greek philosopher, was the first to hypothesize that matter consists of tiny particles he called atomos, the modern understanding of the structure of the atom wasn’t developed until the early 20th century. By this point, scientists understood that every atom is composed of both positively charged particles, called protons, and negatively charged particles, called electrons. What remained unknown was the precise structure of the atom—in other words, how protons and electrons were distributed within an atom.
The theory of quantum mechanics, which originated around the year 1910, provides a great leap in the understanding of atomic structure. The simplest atom is that of hydrogen, consisting of one proton and one electron. For hydrogen, the primary equation of quantum mechanics, known as the Schrodinger equation, can be solved relatively easily, and the solutions can be written down on paper. However, the hydrogen atom is the only element for which such an explicit solution is possible. The Schrodinger equation becomes far more complicated in a quantum system with multiple electrons, so explicit solutions cannot be calculated.
In multiple electron systems, solving the Schrodinger equation gives us access to a wealth of information, such as the band gap. The band gap is a quantity that indicates how well a material conducts electricity; a low band gap suggests that electrons only need to absorb a small amount of energy to participate in conduction, indicating that the material would be a good conductor.
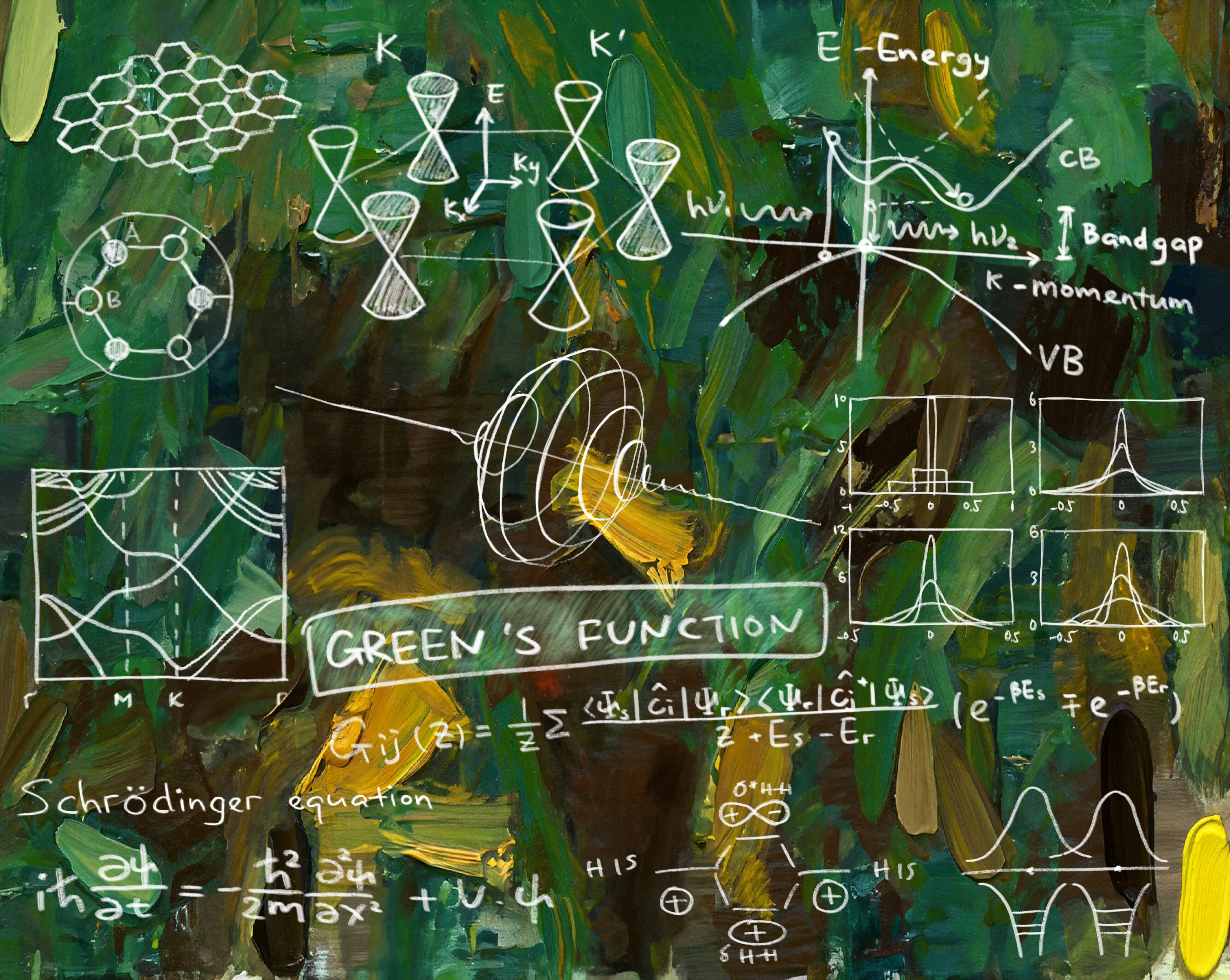
A particularly important example of a multi-electron system is graphene, a form of carbon with peculiar properties, such as high electrical conductivity. Analyzing the Schrodinger equation in materials such as graphene would provide a theoretical foundation for predicting their properties.
Understanding the structure of quantum systems aside from hydrogen is substantially more difficult due to the lack of an explicit solution to the Schrodinger equation. This leap in difficulty from the hydrogen atom to other systems has guided research in quantum chemistry for about a century and is the inspiration for UC Berkeley graduate student, Zhen Huang. “There are two broad kinds of questions I work on. One is to come up with new methods to analyze the equations, and the other is to simplify existing work by speeding up the computations,” explains Huang.
The Schrodinger equation roughly describes the position of an electron in a quantum system. As it is very difficult to solve, understanding the solutions to the Schrodinger equation usually involves utilizing approximations to simplify computations, in addition to mathematical ingenuity. Huang’s research is no exception: one of the problems he considers is classifying Green’s functions for the Schrodinger equation. These functions are akin to building blocks for the Schrodinger equation; once a Green’s function can be computed, a mathematical procedure can be applied to understand the solution to the Schrodinger equation. Huang says, “We discovered that Green’s functions cannot be just any function, but must satisfy certain specific properties.” By narrowing down the properties of Green’s functions, Huang and his coauthors restricted the class of functions that could potentially be Green’s functions. This approach narrowed down where in the quantum system the electron could be, leading to a better understanding of the structure of quantum systems.
Complementing his theoretical work on Green’s functions, Huang also works on more computational aspects of quantum chemistry. He explains that due to the difficulty in calculating quantities exactly on paper, researchers turn to computers to implement numerical algorithms to carry out the required calculations. Many of these algorithms are very intensive, requiring a lot of computational resources and time to be executed. In fact, when they were first developed in the 1950s and 1960s, contemporary computational resources were insufficient to implement these algorithms, rendering them practically useless. “A paper from several decades ago might contain a formula which the authors couldn’t have computed anything with,” Huang points out. Fortunately, advances in technology and computation have vastly enlarged the class of useful algorithms, and previously intractable formulas can now be used.
In addition to increasing computational speeds, the research done by Huang and other members of his lab has practical applications in quantum chemistry. Huang notes that his work could be applied to calculating the theoretical band gap in an oxygen atom. This idea represents a significant step forward in calculating a property that could previously only be measured experimentally.
More broadly, quantum chemistry is essential to understanding the behavior of materials like graphene. Graphene’s electrical conductivity is high—unusual for non-metallic substances—and could potentially be used in solar cells. Theoretical progress made by researchers in Huang’s line of work will be applicable in the study of graphene and related materials. This new knowledge could have impacts on efforts in green energy, as graphene solar panels may be cheaper to produce on a commercial scale.
This article is part of the Fall 2023 issue.