Learning on the fly
Untangling the neural circuitry behind the brain's sense of direction
On a warm summer afternoon, you sit pleasantly at a café nearby—the one with excellent-smelling coffee beans. As you scroll through the latest Berkeley Science Review issue on your phone, something darts before your eyes. You lower your phone and see a small fly has landed on your table. It scurries to the left of the table, delicately rubbing its tiny front legs together briefly, then approaches you before retaking flight. You stand up and start walking south toward the park where you agreed to meet your friends.
Throughout this enchanting encounter, two remarkable brains were at work, engaging in millisecond calculations to make sense of the world around them. Your brain perceived the warmth of the afternoon and the captivating scents of the café, processed visual information from your phone, orchestrated the movements of your body, and remembered the park appointment with your companions. On the other hand, the fly zoomed into your field of vision, landed on the table, ambled about, and quickly retook flight. Despite advances in neuroscience, how exactly brains conduct the calculations needed for these processes still eludes us. At UC Berkeley, researchers are trying to understand brain function by unveiling how cells in the nervous system are connected.
The difficulty is in the numbers
The human body has roughly 100 billion nerve cells, known as neurons. These cells send and receive electrical signals across the body that coordinate all the functions needed to stay alive. However, the large number and diversity of neurons make mapping specific tasks to individual cells difficult. “Neurons are not all the same. Individual neurons have very complicated properties, both electrical and chemical,” explains Dr. Yvette Fisher, assistant professor in the departments of Neurobiology and Molecular & Cell Biology. To make matters even more complicated, neurons don’t act as individuals. They are connected by intricate neural circuits that carry out specific tasks, such as movement and learning.
Neural circuits occur between all types of neurons and can connect to form large-scale networks. “You can think of them as an electrical circuit on your phone’s motherboard," says Lily Nguyen, a PhD student in Fisher's research group. "Individually, each part of the circuit has a role, but only when they come together can you have a functional device." The group tries to understand neural circuits by studying fruit flies, which have around one million times fewer neurons than humans. This smaller number of neurons makes them a useful model organism for studying the nervous system. Nguyen adds, “A lot of the fundamental components of the fruit fly nervous system are similar to humans. Their neurons are made of the same components and make the same types of connections as humans.” Flies are also easy to work with practically—they breed readily in captivity, and a large arsenal of genetic manipulations is available to study fly physiology. The Fisher group uses fruit flies to understand the neural circuitry involved in one specific brain behavior: sense of direction.

Informing the compass with visual cues
A brain’s sense of direction allows it to keep track of an organism’s body, even when external cues are lacking. “Imagine sitting in your room and closing your eyes,” Fisher says. “Your brain still knows where you are in the room, even though you can’t see. If you were to move around with your eyes closed, your brain would understand where your body is moving—forward, left, backward. It’s an internal sense.” Sense of direction is also dynamic; other senses can update it in the brain. “If you were to open your eyes, your brain immediately would use the visual cues in the room to realign your sense of direction,” Fisher adds. That means the brain constantly manages an interplay between spatial awareness and visual learning. Fisher studies this interplay in a region of the fruit fly brain known as the ellipsoid body.
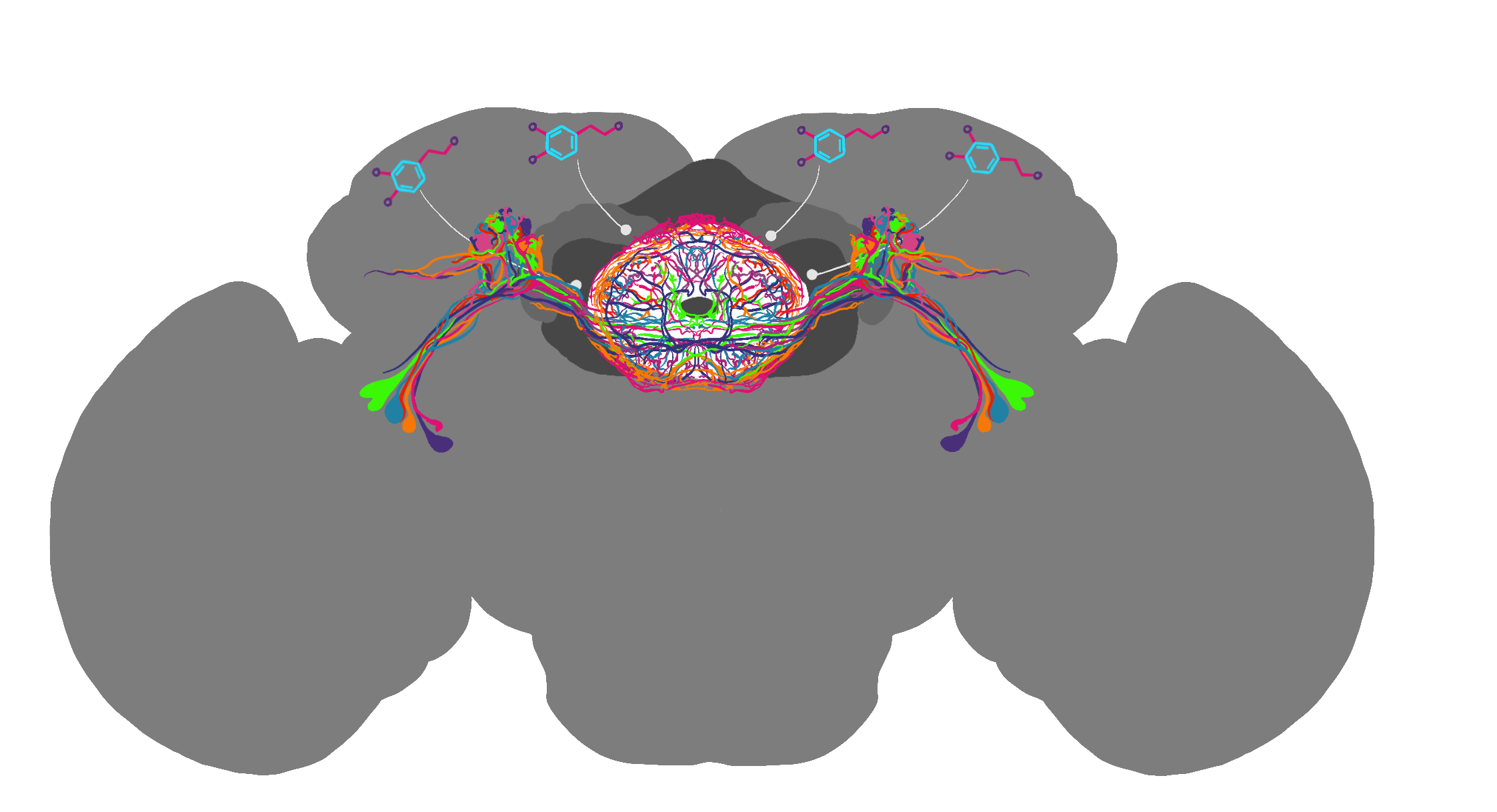
A ring-like structure that lies in the center of the fruit fly’s brain, the ellipsoid body acts as the fly’s internal compass. Researchers can track activity in the ellipsoid body by measuring calcium levels in neurons. When neurons communicate, the electrical signals they send and receive are accompanied by increasing calcium ions in the nerve cell. Fisher's group uses a specialized imaging technique called two-photon microscopy to track the calcium levels in live fruit fly brains. “We take the fruit fly and place a small probe in their head, which allows us to use microscopes that shoot light into their brains and measure neural activity,” explains Fisher. “We can also use electrical probes to measure neural activity of specific neurons while the fly walks on a tiny foam ball floating in the air.” This makeshift fly treadmill allows the researchers to track the direction of the fly at the same time as they are tracking neural activity. They place the flies on their floating balls in small boxes with panoramic LED screens, allowing the researchers to animate stimuli around the animal. “It’s almost like they’re playing a boring VR video game,” Fisher jokes.
Using this technique, scientists observe that as a fly turns its head, activity shifts from one place to another in the ellipsoid body, almost like a needle in a compass. Only a specific portion of the ellipsoid body has high activity at any given time. Scientists believe this high activity is the brain’s way of tracking the fly’s body orientation relative to its surroundings. But how does the brain maintain and update its sense of direction according to environmental changes?
In a recent publication, Fisher studied how fruit flies navigate their surroundings using visual cues and self-motion information. Specifically, she focused on compass neurons found in the ellipsoid body. These neurons use data from the fly’s movements to estimate its sense of direction, even without visual cues. When visual cues are available, such as landmarks or objects, the neuron activity in the ellipsoid body becomes more stable, tethering the fly’s sense of direction more clearly. Fisher discovered that visual cues are integrated into the compass neurons through inhibitory neurons called R neurons (or ring neurons). Specific visual cues can evoke inhibitory signals from R neurons onto compass neurons.
Interestingly, some visual cues cause the R neurons to become more active. For example, single and distinct visual cues cause a more robust inhibitory response. One example of such a cue could be the Sun. It always looks like it's very far away, making it a reliable reference point for navigation. This tunability of sense of direction suggests that the connections between R and compass neurons are not identical; some connections are more substantial, and others are weaker. More excitingly, the study also showed how visual cues affect the compass neurons, which can change over time. The compass neurons adjust their responses to match the new surroundings as the fly explores environments with different visual cues. These changes in neural connections help the fly maintain a coherent sense of direction in an ever-changing environment. Using visual cues and adjusting their responses could be essential for many animals, not just fruit flies, to find their way around in their surroundings.

Turning on a dopamine dime
While Fisher's previous findings demonstrate that visual cues are connected to the brain's sense of direction, they don't explain how the brain chooses when to modify its sense of direction. One answer seems to lie in an unsuspecting type of neuron in the fly’s internal compass: dopamine neurons. “The canonical role of dopamine is usually in the context of reward learning. When an animal is trying to learn how to do something—if it's something rewarding—their dopamine neurons will be very active,” Fisher explains. Her recently published work shows that dopamine neurons in the fruit fly ellipsoid body are selectively active when the fly turns. Dopamine release from neurons strengthens the association between visual cues and head direction cells. This association is crucial for the fly to form a stable map of its head direction in response to the environment. “For the neurons in the navigation circuit trying to tell where you're facing, it's best to learn when you turn. That is when you see a new view, which is important to process for a sense of direction.”
When dopamine release is suppressed, the fly's ability to associate visual cues with its head direction is impaired, further proving the role of dopamine in spatial learning. This type of impairment is appropriate, for example, when objects enter your field of view. “If you were standing in your room right now and a bird flew into your field of view—that doesn’t tell you anything about where you are. So, it's advantageous not to stimulate a change in direction when you're still and only to learn when you move.” Regulation of sense of direction by head turning could be just the tip of the iceberg. Flies can also perceive the wind’s direction and the light’s polarization, leading to more unanswered questions regarding the possible regulating mechanisms behind sense of direction.
From neurons to self-driving cars
The intricate neural circuitry behind fly navigation and sense of direction continues to captivate Fisher and the group. Despite its simplicity compared to the human brain, the fruit fly’s brain is a valuable model for investigating fundamental neural function principles. By studying neural connections in the fruit fly brain, her group seeks to answer profound questions about the nervous system. “How are neurons communicating with each other to form circuits?” Nguyen comments. “How are these circuits allowing them to perform the complex computations that you need for behaviors like sleeping, eating, and even thinking? These are all fundamental questions we hope to bring answers to.” The diversity and complexity of neurons present challenges, but they also open exciting avenues for exploration. How does the brain choose when to modify its sense of direction based on varying stimuli? What other mechanisms regulate sense of direction in flies, such as perceiving wind direction and light polarization? Uncovering the answers to these questions could provide valuable insights into fly navigation and broader aspects of neural processing and behavior in diverse organisms. Their work could even prove helpful in developing algorithms for computations.
Algorithms, which serve as the backbone of artificial intelligence (AI), share striking similarities with how the brain processes information. Just as neurons in the fruit fly's brain communicate and form circuits to enable learning and decision-making, AI models learn patterns from data and make data-driven decisions. Fisher highlights this potential synergy: "Brains are exactly how you'd want to do machine learning. You teach a 'circuit' something and need a teaching signal telling the computer whether it's correct." One exciting application of this synergy is in the development of autonomous vehicles. By emulating the brain's remarkable ability to adapt to changing environments, AI systems could become more robust and capable of safe navigation in complex real-world scenarios. Like how a fruit fly's brain processes information from its surroundings to navigate, algorithms can be imbued with similar mechanisms, enhancing autonomous vehicles' perception and decision-making capabilities. Imagine a self-driving car that learns from its environment, just as a fruit fly does. It adapts to new road conditions, recognizes and responds to traffic patterns, and continuously refines its navigation skills, all based on real-time visual cues. By incorporating insights from fruit fly research, AI-driven self-driving cars could become safer and more efficient on the road. AI models of many kinds can be improved upon by harnessing and incorporating the brain’s computations and ability to dynamically adapt. The discoveries made by Fisher and others at UC Berkeley also hold profound implications for human health and medicine. While their work primarily focuses on the intricacies of neural circuitry in fruit flies, their findings can potentially translate into a better understanding of human brain function. Unraveling the mysteries of neural circuits could offer insights into neurological disorders and conditions that affect sense of direction, spatial awareness, and learning. By leveraging the knowledge obtained from studying simpler organisms, scientists may find new avenues for treating navigation and spatial cognition disorders in humans.
Moreover, studying dopamine neurons and their role in modifying the brain's sense of direction provides valuable insights into its reward and learning systems. This understanding could contribute to advancements in treating addiction, depression, and other mental health disorders that involve dopamine dysregulation. Applying the principles of neural modulation and learning mechanisms could lead to innovative therapeutic strategies targeting these disorders. As UC Berkeley scientists delve deeper into the inner workings of the nervous system, they inch closer to deciphering the neural basis of fundamental animal behaviors and unlocking the brain’s countless secrets.
This article is part of the Fall 2023 issue.