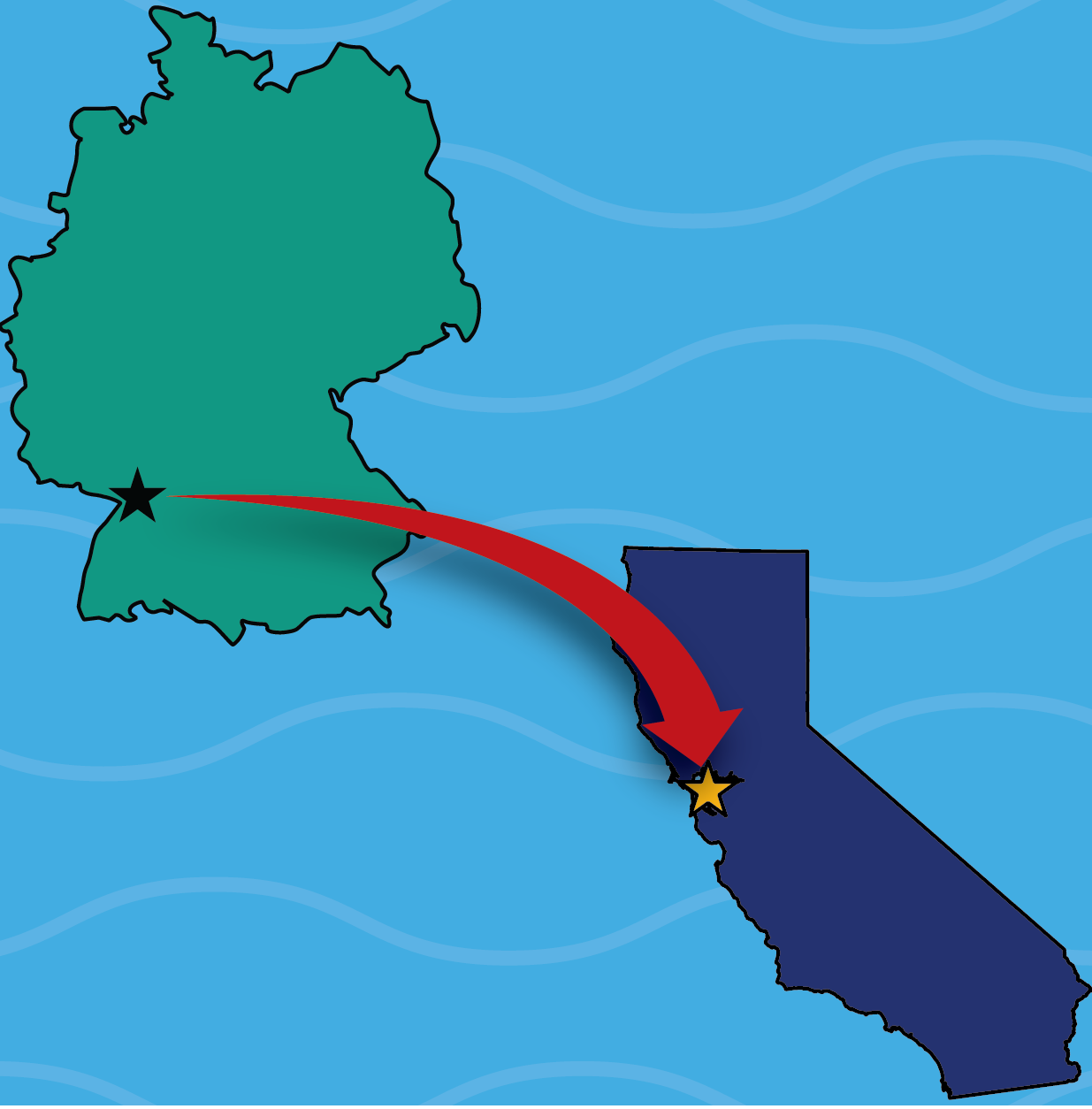
From the Field: Working against an atomic clock
It is 8 a.m. and I haven’t had coffee—caffeine makes my hands shake, and today I will be picking through delicate crystals to bring to the Advanced Light Source (ALS) for single-crystal X-ray diffraction. Crystals are very important to the field of inorganic chemistry. Crystallization is used as a purification method as well as to unambiguously characterize what you made—the process of bombarding a crystal with X-rays, collecting the diffracted X-rays, then using a lot of math, produces an image of the molecule that is the closest thing we have to “looking” at atoms. I am accustomed to working with crystals that decompose in air and am comfortable handling even the most persnickety and delicate samples. But today, the crystals aren’t just reactive with air and water: they are radioactive, meaning that they will also be falling apart from the inside. I haven’t had any coffee, the clock is ticking, and every minute we don’t get the crystals to the ALS is an opportunity for this precious material to crumble.
Relatively few crystal structures containing radioactive metals have been reported, and of those, even fewer are air-sensitive. The crystals I am working with today are especially important to the institution where they’ve been synthesized. I am a postdoctoral researcher in synthetic heavy element chemistry at Lawrence Berkeley National Laboratory and I work collaboratively with UC Berkeley graduate students in my research. Our lab, the Heavy Elements Research Laboratory (HERL), is just a short walk up a steep flight of stairs from UC Berkeley, where Glenn T. Seaborg, Joseph Kennedy, and Arthur Wahl discovered the element I am working with today: plutonium. Seaborg and co. isolated plutonium-238 in an attic laboratory of Gilman Hall in 1940. Though Gilman Hall no longer houses active research labs, you can still wander through the building, go up the large spiral staircase, and see the door to the room where plutonium was first isolated. Several plaques are positioned outside the small wooden doors, designating the laboratory a nuclear historical landmark by the American Nuclear Society and a registered national historic landmark by the U.S. Department of the Interior.
It’s 8:15 a.m., and I’m thinking that almost 83 years after its discovery, plutonium and its unique chemistry—with applications beyond defense, including sustainable energy and forensics—are some of my main areas of study. The crystals we’re working with on this overcast morning are a deep burgundy red; they are organometallic, meaning they contain metal-carbon bonds, which often results in vibrantly colored materials. It has been a long road to arrive at these high-purity crystals. Starting from a heritage stock solution—as radiochemists at Berkeley Lab, we are stewards of a wide variety of isotopes, due in part to the history of the elements’ discovery here—the desired isotope must be separated from its radioactive decay products, or “daughters,” before use. These daughters, named in the feminine out of reverence for pioneering radiochemist Marie Curie, must be painstakingly removed from the plutonium via a series of chromatographic separations.

Once pure, the isotope is “dried” to be used as a starting material in our chemistry. This chemistry is performed in specialized gloveboxes that are outfitted with a variety of engineering controls that keep us and the environment safe, including thick rubber gloves that allow us to reach inside the box to handle our samples. Today, we have synthesized a new plutonium-containing organometallic molecule and grown large, high-quality crystals that we intend to take to the ALS. We only get to go to the ALS a few times a year, so everything—isotope purification, synthesis, crystallization—is meticulously timed so that the crystals are ready on exactly the day that we have ALS access.
It’s 8:30 a.m., still no coffee, and I am picking out blood-red crystals and placing them one by one in oil on a microscope slide to try and pick out something suitable to send to the ALS. I am working through three layers of gloves: the first layer is long and taped over my lab coat, the second is the rubber gloves of the glovebox, and the third is layered on top of the rubber gloves inside the box to avoid contamination of the glovebox gloves. My dexterity is limited by the gloves, the height of the glovebox holes, and the ever-present fear of spreading plutonium contamination in the box—if there is radioactive material anywhere other than the crystal, I will have to decontaminate the crystal holder once it is outside the box. More time spent messing around with the crystal when it is outside the box means a higher probability of it decomposing before we can get it to the ALS. It is a high stakes game of radioactive Operation, and it is thrilling.
I think about the naming of plutonium as I place the crystal on its holder and wait for the glue that keeps it in place to dry—we don’t want such a precious and radioactive sample to be misplaced! Apparently, the old attic lab in Gilman Hall was nearly unusable after the isolation of this new element due to the stench, and Seaborg cheekily made the symbol for element 94 “Pu” as an homage to its unpleasant odor. Separated as I am now from the plutonium by thick plexiglass shielding, I can’t help but wonder if this new organometallic also smells bad. Hopefully, I will never know.
I can only bring one crystal at a time to the ALS, so I try to pick the best one. As I wait for confirmation that the sample I’ve brought out of the box is not contaminated, I look back at the microscope slide and see that the crystals are beginning to dissolve in the oil. Now we really need to rush. It is 10 a.m., and I take my plutonium on a 10-minute walk farther up the hill to beamline 12.2.1 at the ALS (you aren’t allowed to take radioactive materials on the shuttles, probably a good rule). The crystal is in place, the beam is operational (not always a given!), and we wait with bated breath to see the first few spots that indicate diffraction. We see the first few data points blink across the screen, and I zoom in for a closer look. Devastation—it’s a “twin.” “Twinned” crystals are two crystals that have fused together, and for math reasons, are very difficult to solve into reliable structures. Deep breath. There are plenty more crystals in the sample, so it’s 11 a.m. and back to the HERL for me.
It’s 11:45 a.m., I am on my third crystal of the day, and I have picked one that looks the best so far and will hopefully give us a structure. I walk it back up the steep stairs, the tiny crystal that took months of planning to make clutched in my hands. I get the crystal in place and we try not to blink as the first few spots appear on the screen—it diffracts! And it’s a high-quality crystal! We are going to have a crystal structure of a transuranic organometallic, and now it’s 12:30 p.m.—I can finally have some coffee.
This article is part of the Spring 2023 issue.