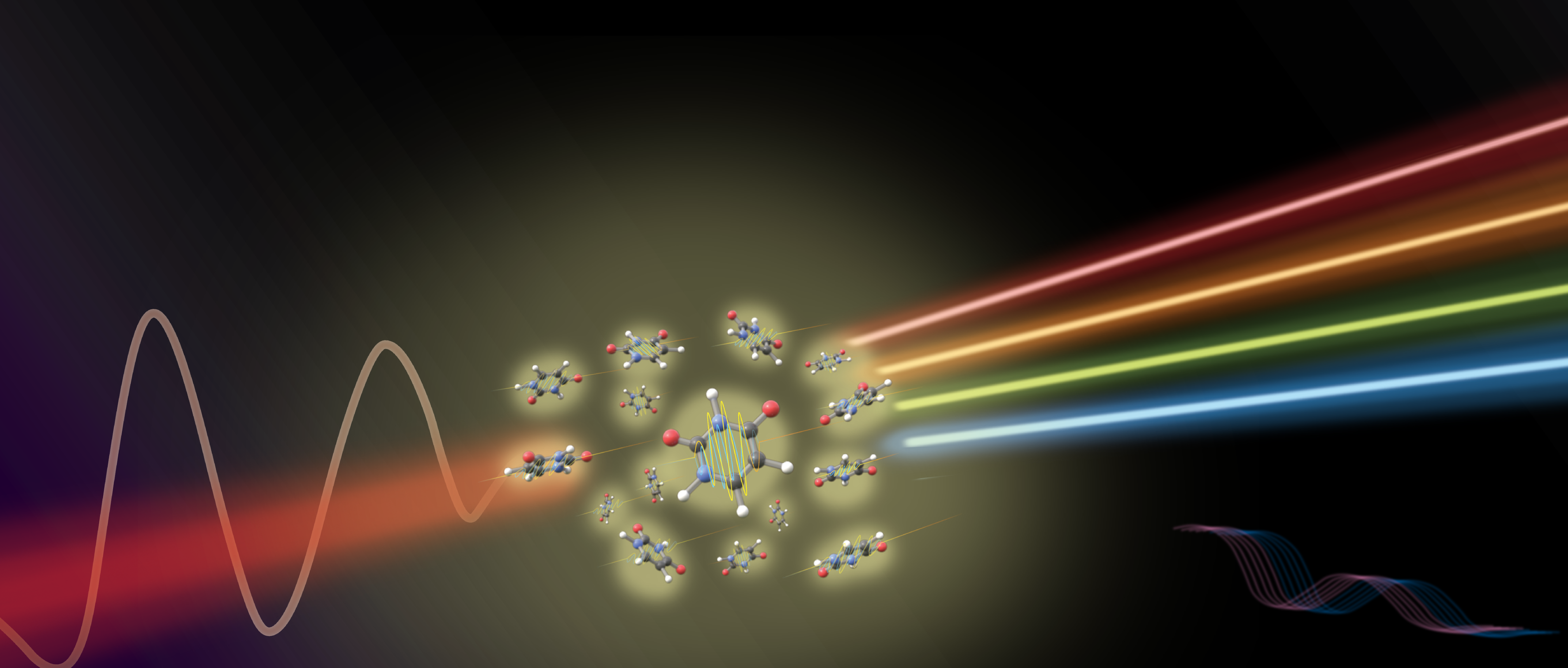
The properties of some of the most useful materials, such as those found in semiconductors and solar cells, depend upon their arrangement of electrons. Thus, to understand what makes materials useful for any particular purpose, scientists need to be able to study how electrons arrange themselves between atoms and how they rearrange when a chemical bond breaks or forms.
In practice, watching electrons move is very difficult because they move on some of the fastest timescales conceivable—on the order of attoseconds (10-18 seconds) to femtoseconds (10-15 seconds). To put that into perspective, those timescales are to a second as a second is to the age of the universe. Past approaches to studying electrons at these timescales involved large facilities like synchrotrons or x-ray free-electron lasers, which are expensive to maintain and limited in the number of users they can accommodate. However, there are new laser techniques that scientists, including many in the Leone, Neumark, and Zuerch groups at UC Berkeley, can use to watch these dynamical processes, such as using a tabletop laser system.
To watch the motion of electrons in real time, many groups at Berkeley use a technique called high-harmonic generation. This approach entails taking an already short laser pulse and making it shorter by making the range of different wavelengths of light it contains much wider. Doing so takes advantage of the principle of time-energy uncertainty, or Heisenberg uncertainty principle, that states that the less certain you are in the energy of something, the more certain you can be of the time something happens. For laser pulses, this principle suggests that the broader the spectrum of light in the pulse, the shorter the pulse can be.
To generate the necessary short pulses, the high-harmonic generation technique relies on the fact that light is an oscillating electromagnetic field. If focused strongly enough, the electromagnetic field of the laser pulses can pull electrons away from the atoms before turning them around and slamming them back into the atoms. When the electrons recombine with the atoms, the excess energy the electron gained while accelerating in the laser field is released as a single photon ranging anywhere from ultraviolet to x-ray wavelengths. Using the broad spectrum of wavelengths emitted during electron recombination, both the number of electrons around an atom and the arrangement of electrons in a material can be observed in real time by monitoring how the material changes the way it absorbs the light.
Using these ultrabroad, ultrafast laser pulses, scientists can observe the way molecules and materials absorb this generated light differently on the fastest timescales imaginable. "These incredibly short flashes of light let us track the movement of electrons in matter on their natural [attosecond] timescale, allowing us to watch chemistry as it is happening live,” remarks Dr. Lorenz Drescher, a postdoctoral researcher in the Leone group at UC Berkeley. “We hope that these insights will lead to a better understanding of how the interplay between many electrons influences the dynamics we see and how these effects can be exploited." After a short laser pulse initiates a chemical reaction, the exact motion of electrons and the rearrangement of bonds can be studied, paving the way for the discovery of new fundamental properties of novel materials. Better yet, these laser pulses can be generated in a small laboratory setting compared to the expensive and in-demand synchrotron and x-ray free-electron laser facilities that were relied upon to do similar experiments in the past. These advances will allow scientists to investigate a wider range of new materials in a more diverse set of labs, leading to not only a growth in our fundamental scientific understanding of these ultrafast processes but also paving the way for faster electronics, more efficient solar panels, and more.
------- Zachary Helm is a graduate student in chemistry
Design by Zhizhi Kong
This article is part of the Fall 2022 issue.