Plants do the wave
Using quantum mechanics to explain the unique efficiency of photosynthesis

Photosynthesis, from the Greek words for “light” and “putting together,” is the foundation for life as we know it. Photosynthesis is the reason our skies are blue and our forests are green, and plant-based agriculture is essential to human civilization. According to the traditional understanding of photosynthesis, the sun sends light to Earth, and this light is absorbed by light-harvesting complexes made of chlorophyll—the molecules that make leaves green. The energy is then transferred from one chlorophyll to another until it reaches the reaction center, where the energy from the sun is used to split water molecules into protons, electrons, and oxygen gas. The protons and electrons then go on to turn carbon dioxide into sugar. Some people might remember this equation from their school days:
6CO2 + 6H2O + light → C6H12O6 + 6O2
However, the processes behind photosynthesis are not as simple as the above definition might make it seem. While we know how much carbon dioxide and water are needed to make a single molecule of sugar, light is a less understood ingredient. Studies are ongoing about how light interacts with photosynthetic systems and how that energy is utilized in the system. Only about 10 percent of photons that hit a chlorophyll per second are absorbed. In contrast, the current efficiencies of solar photovoltaics, like those used in our solar panels, range from approximately 10 percent to 25 percent, with some experimental studies going so far as 46-percent efficiency.
The relatively low absorption efficiency of plants is a direct result of the properties of sunlight. Sunlight is a weak source of light and has a broad, uneven distribution of wavelengths, including low-energy infrared, the intermediate visible colors, and high-energy ultraviolet. Only some of those can actually be utilized by the plant. Additionally, plants have adapted to hour-to-hour changes in light conditions, like moving clouds, by maximizing the rate of photosynthesis at low light conditions. Consequently, reaction centers can be closed for business when too much light is available, further decreasing the amount of absorbed light.
Even so, most scientists describe plants as very efficient, because of how little energy is lost after it is absorbed, when it’s being transferred from one chlorophyll to another on its way to the reaction center. Plants have near perfect “quantum” efficiency; almost 100 percent of absorbed photons give rise to stable products (i.e., oxygen gas, NADPH and ATP, with the latter two being used to power the sugar making process). Long-held assumptions about how energy gets from the initial absorbing molecules to the reaction centers where chemistry occurs do not explain the efficiency with which photosynthetic organisms complete the process. In UC Berkeley’s College of Chemistry, Professor Graham Fleming and Professor Birgitta Whaley’s groups are researching the fundamentals of how these systems absorb light and transfer that energy—and how they do it so well. As it turns out, quantum mechanics may play a key role in the process.
Small scale, big effects
Classical mechanics, which describes the motion of a macroscopic object like a ball rolling down a hill, breaks down at extremely small sizes. For instance, classical mechanics cannot be used to describe how electrons move around the nucleus of an atom. At the atomic level, quantum mechanics comes into play. Specifically, there are three important principles in quantum chemistry: wave-particle duality, the uncertainty principle, and the Born-Oppenheimer approximation. Wave-particle duality states that all small objects can exist as a wave and a particle simultaneously, though we usually think of waves as disturbances that act on or move through particles, like a sound wave through the air. The uncertainty principle describes how, as a consequence of wave-particle duality, the location and movement of an object cannot be known precisely. The Born-Oppenheimer approximation, which assumes that the atomic nuclei and electrons in a molecule can be treated separately, is used in quantum chemistry and molecular physics to simplify calculations by getting rid of any interactions between the nuclei and the electrons.
Although we typically use these principles to understand the behavior of small objects like atoms and electrons, technically objects of any size could be described with quantum mechanics. However, for objects of larger size or greater energy, quantum mechanics begins to match classical observations, and it is easier to use well-understood classical mechanics for your formulations. For example, the uncertainty principle states that an object can never be at rest; otherwise, you would know both the location and the speed of the object simultaneously and precisely. While this observation is crucial to our understanding of how electrons move around atoms and molecules, it becomes little more than a novelty when we think about a baseball, for which the uncertainty is so small that we readily observe it at rest. Since classical mechanics is a lot easier to calculate, you would not use quantum mechanics to explain the movement, or lack thereof, of a baseball.
While it’s obvious you would use quantum mechanics to describe a single electron and classical mechanics to describe a baseball, many biological phenomena, like photosynthesis, occur at the molecular level, where many atoms may be interacting, and are thus at the edge of classical and quantum mechanics. Scientists who study such phenomena often tend to use simpler classical mechanics when possible, but that’s not to say that scientists never consider quantum mechanics in the context of biology. After all, biologists have long understood that the principles of quantum mechanics play a central role in the light absorption part of photosynthesis; light with a sufficient amount of energy excites an electron in a chlorophyll molecule to a higher energy state. In fact, the descriptor “quantum biology” was coined by Erwin Schrodinger all the way back in 1944, in his book What is Life? The Physical Aspect of the Living Cell.
However, as a biological phenomenon becomes more complicated, the value of considering quantum mechanics becomes less obvious. As Dr. Robert Cook, a postdoc in Dr. Whaley’s group, explains, “when you get a lot of atoms together, enough to build a biologically important molecule, the quantum mechanical equations become too complicated to solve exactly, but also the quantum mechanical effects can become less important.” Quantum mechanics explains how a chlorophyll molecule gets excited to a higher energy level, but it isn’t typically used when we talk about a graduate student getting an influx of energy from coffee. Still, just because a system can be described using classical mechanics, does not mean that quantum mechanics should be entirely ignored.
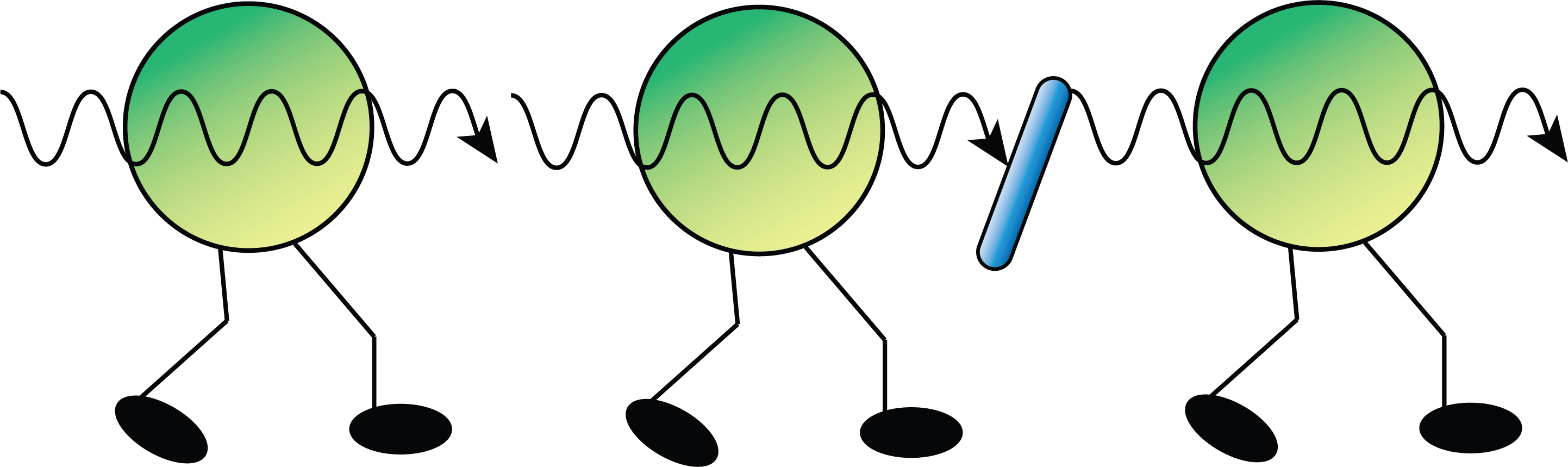
For instance, the transfer of light from the site of absorption to the reaction center during photosynthesis is often described by biologists in a way that ignores some aspects of quantum mechanics. They might say that the energy from light is transferred from chlorophyll to chlorophyll in the same way one would throw a frisbee. The frisbee is always given whole and only one person has their hand on it at a time. However, this description introduces room for error and fails to account for the quantum efficiency of photosynthetic organisms. A more quantum mechanical explanation would be that chlorophyll molecules are runners in a relay race passing a baton. Initially, one runner has the baton. When the first runner is trying to give it to the next runner, there is a moment when both runners have their hands on it. Technically, if the objective was just to move the baton, they could throw the baton to each other. However, it is a lot easier to make sure the other person has a hold on it before you let go, otherwise you risk dropping the baton in the process. As nice as this quantum mechanical explanation sounds, it was only with the more recent breakthroughs in laser technology that phenomena like energy transfer could be experimentally observed.
A quantum biology revolution
Many quantum interactions are very fast and are thus very hard to see in an experiment. Think about how you would capture a hummingbird’s wings on camera. Hummingbirds beat their wings about 200 times per second and the average camera shutter speed is only 1/60 of a second. If you tried to take a picture at that shutter quality, you would only get a blur. You would need a shutter speed of around 1/1,000 or 1/2,000 of a second to be able to clearly capture a hummingbird’s wings in motion. Quantum processes are even faster than a hummingbird’s wings. These effects are often so fast that you can’t see them with a camera—or any electronics. Instead, scientists use lasers.
A laser is a device that generates “coherent” light by exciting a material until it spontaneously emits light. That light, which initially consists of a range of wavelengths and energies, is then reflected continuously between two mirrors, interfering constructively and destructively with itself until only one, amplified energy of light remains. The remaining light is special because it is spatially coherent—it can be focused and can be kept the same size over a great distance—and temporally coherent—it can have a very narrow energy range (continuous wave laser) or be used to create pulses of light (pulsed laser). In past three decades, advances in technology have made it possible to shorten these pulses of light to extremely short durations (on the scale of femtoseconds or 10-15 seconds). Because such short time periods are crucial to study key processes in quantum biology, the ability to prove the importance of quantum mechanical effects in biological systems didn’t exist until femtosecond laser technology was invented. While the advent of femtosecond lasers led to the ability to “see” fast processes, using ultrafast techniques to answer questions about the fundamental processes in biology was still a big leap, since biological systems are very difficult to work with compared to typical samples studied with ultrafast optics. “Biological systems are warm, wet, and noisy,” according to Fleming. “These are not the ideal circumstances to observe delicate quantum mechanical effects.” Sorting out the quantum mechanical effects in something so complicated would mean creating new techniques.
To investigate the physics of biological systems—in this case, photosynthetic bacteria—the Fleming group used an ultrafast laser technique called 2-Dimensional Electronic Spectroscopy (2DES). This technique uses multiple laser pulses: one pulse, called the pump, prepares the sample by putting it in an excited state and another pulse, called the probe, monitors the changes caused by the pump. Both of these laser pulses have a wide range of energies so that they can excite and track multiple excited states, which is good for such a messy system. Excitation states are like rungs on a ladder; to know which rung you are on, you have to have a wide enough range of energies to examine as much of the ladder as possible. For example, if we see signal at one energy on the pump axis and a different energy on the probe axis then we know that the energy moved up or down the ladder in the time between the pump hitting the sample and the probe hitting the sample. 2DES can track that energy movement by taking measurements over time. The paths the energy takes through the system and how long it takes can tell scientists a lot about how a system of interest interacts with energy.
The 2DES experiments in the Fleming group were able to show that the energy started in one chlorophyll but instead of being “thrown” to the next, it was shared between the two. The experiments showed a phenomenon called “quantum beating,” which arises when energy is being shared by both molecules rather than existing only at one or the other. Scientists call this type of energy transfer “wave-like” because you must take the wave part of wave-particle duality into account for this superposition to occur. The molecules had shared possession of the energy during the transfer for a time before that energy settled on the accepting molecule. This energy sharing was surprisingly long-lived—about a thousand times longer than the femtoseconds usually associated with quantum phenomena. Scientists in the Fleming group thought that this may be how photosynthetic systems prevent a lot of energy loss; the “baton” is being passed without being dropped because both “runners” have their hands on it for a time.
New questions need new ways of answering
However, this concept was not without its controversy. “There were people who thought results with femtosecond lasers had nothing to do with what happens in sunlight, that it was simply irrelevant. Even if we saw these fancy oscillations with our femtosecond lasers, it had nothing to do with how plants actually worked,” Fleming explained. “And then there were people who were a little bit more subtle and started to worry about how vibrations might come into this.” Different amounts of energy interact with different parts of a molecule. While heat in a room can cause a molecule to move and microwaves can cause it to rotate, more energy is needed to cause the molecule to vibrate. Exciting an electron to a higher energy would require visible or ultraviolet light and ejecting an electron from the atom entirely would take even more energy, like x-rays. The observed quantum beats could have been inherent to the behavior of the nuclei (vibrational), the electrons (electronic), or a result of interactions between the two. The 2DES technique only focused on the electronic energy levels, so Fleming tasked his students with creating a new way to look at vibrations in the system—2-Dimensional Electronic Vibrational (2DEV) spectroscopy. Instead of using visible light as the pump and the probe which could only interact with the electronic states of the system as in 2DES, 2DEV used a visible pump and an infrared probe so that it could study both electronic excitations and vibrations. By simultaneously looking at vibrations and electronic excitations, the team confirmed the wave-like energy transfer first seen in the 2DES experiments. But this new experimental technique could also tell scientists something new: the wave-like energy transfer was a result of vibronic coupling, a portmanteau of “vibrational” and “electronic.” Scientists were able to see that some electronic energy gaps matched vibration frequencies in the experimental data, leading to the observed quantum beating. This vibronic interaction represents a break-down of the aforementioned Born-Oppenheimer approximation.
Some skeptics continued to argue that the quantum nature of the energy transfer was a result of how the systems were being studied. According to Fleming, “it became clear that, whether a system evolves quantum mechanically—truly quantum mechanically—is probably not answerable with conventional laser techniques.” Perhaps these data were a result of using lasers in a lab, rather than observing a plant in a field using sunlight.
“Lasers have become an indispensable tool for studying all kinds of things,” explains Cook. “However, there are key differences between sunlight and a laser, and it's currently an open question if these differences matter.” Lasers are more intense than sunlight—almost 1 million times more intense than average daylight. Unlike the sun with its broad, uneven distribution of wavelengths a laser consists of a very narrow range of wavelengths. To solve this problem, Fleming needed yet another new technique, one where the use of a laser as the light source would be irrelevant: single photon studies.
Quantum mechanics gains its name from the fact that some things are “quantized” or that they have discrete values rather than the continuous values seen in classical mechanics. Thanks to wave-particle duality, we can treat light as a single particle when doing single photon studies. These single particles have a discreet energy and thus don’t have a wavelength range. We can also control the amount of light that the system sees by sending in more or fewer single photons so that the intensity of the light is more comparable to what a chlorophyll in a plant would see.
The Fleming group uses a special crystal to generate an entangled pair of photons, one pair at a time. This pair is easily separated because they oscillate in different directions and can thus be split with a polarizer. While one of the photons goes on to interact with the sample, the other photon acts as a herald. The herald arrives at one of the detectors first and says, “Hey, look, the signal is coming shortly!” to the computer, which now knows that any signal received at the second detector is a result of the experiment. If there is no herald photon within a limited amount of time, then any signal received is discarded. There must be signal at both detectors practically simultaneously for the signal to count, which is way more statistically likely if the signal at the detectors came from the photon pair. In this way the detectors have an easier time separating the actual signal from the background light in the room.
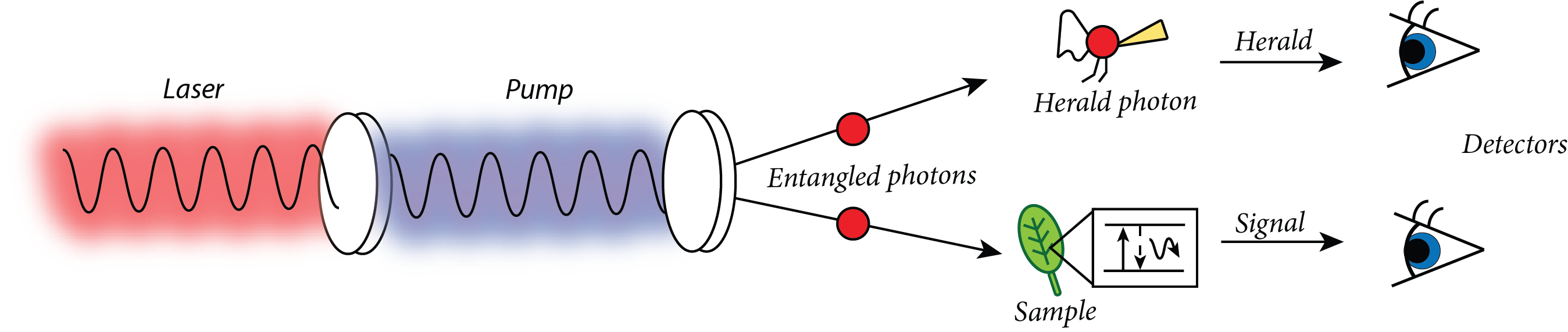
The first single photon experiment on a photosynthetic system involved light harvesting complexes from bacteria. When they sent the photon in, the scientists saw a quantum jump—the photon was “swallowed” whole. This was the first experimental proof for how photosynthetic systems absorb light on a single photon level, and it showed that the intensity of the light and the wavelength range of the light likely do not matter to the molecules absorbing light, because they would only be absorbing one photon at a time. Thus, it is not likely that there is a measurable difference to how the system behaves when absorbing sunlight or laser light. The Fleming group has no intentions of resting on their laurels, though. Now that they’re working with Professor Whaley’s group to see just how complex their explanations can get. Dr. Cook elaborates further, “We are working on what role an open environment plays in the absorption of single photons.” In an open environment, the system of study can interact with, and lose energy to, its surroundings as vibrations. “Interestingly,” Cook explains, “the energy lost to the environment also contains information about the internal dynamics of the system.” While adding a place for the energy to dissipate to is more accurate, it also can dampen any signs of quantum coherence in the model, in much the same way adding more and more molecules can hide the underlying quantum dynamics.
What’s the worth?
Scientists hope that by tapping into quantum phenomena, they can tweak current plants to grow faster and more efficiently. “We want to have the whole set of tools in our toolbox, and we don't know whether we do or we don't,” Fleming explained. One day we may be able to manipulate the quantum mechanics of a system—biological or artificial—as easily as we manipulate mechanical properties like elasticity or strength. Quantum biology research could be applied to agriculture, energy cultivation, and even production chemistry. Cook is particularly hopeful with regards to solar cells and energy harvesting: “By studying photosynthesis at the fundamental limit of a single photon, we will be able to unravel the source of this efficiency and we can leverage our knowledge for cheaper and more efficient solar cells.”
“We're constantly learning lessons from nature, which are stimulating synthetic devices,” says Graham Fleming. “But these devices won’t look the same, just as planes don't fly like birds fly, but birds showed us it was possible.” For example, while it is unlikely that we will see fully artificial plants, we could replicate the wavelike energy transfer physics to increase the transfer and storage efficiency. The National Science Foundation’s Center for Synthesizing Quantum Coherence, a collaboration by professors and their groups at UC Berkeley, Duke University, University of Illinois, Urbana-Champaign, and Northwestern University, have built their center around the idea that scientists can mimic the coherence found in photosynthetic complexes in new and interesting materials, materials that could be used for solar energy conversion. While much progress has been made, and the ideas inherent have spread outside of UC Berkeley, there is still a lot of work to do. “Science never ends,” Fleming explained. “We did one experiment that sounded like we'd solved the problem, but then issues were brought up, so then we devise another experiment, but still there are unresolved issues and we have to come up with another experiment.” For Graham Fleming and his group, there is still a lot of work to do studying the fundamentals of photosynthesis. As for whether they are on homing in on a consensus, a singular answer, Fleming replied, “I hope so, but that's not up to me to decide.”
-------
Kaydren Orcutt is a graduate student in chemistry.
Designs by Abby Estes
This article is part of the Fall 2021 issue.