Tiny brains, huge gains
3D cell culture transforms science and medicine
The brain is the most complex organ of the human body; the three-pound mass of fat and protein controls everything from the beating of our hearts to the storage of our memories. All of these functions depend on the brain’s interconnected network of neurons, which are cells that send and receive electrical and chemical signals throughout the brain and other organs of the body.
The transfer of information through neural connections allows us to think, feel, move, and comprehend the world around us. But how exactly do 80 billion neurons and their 100 trillion connections orchestrate our movements and thoughts? And what happens when neurons and their connections are disrupted or fail to properly form in the first place?
These questions are still mysteries, but tiny lab-grown clusters of brain cells, termed brain organoids, may reveal the answers.
What are organoids?
An organoid is a three-dimensional (3D) structure that resembles a particular human organ, but in its most rudimentary form, an organoid is really just a ball of stem cells grown in a lab. Stem cells have the unique ability to turn into various types of cells through a process called differentiation. To control what type of cell a stem cell becomes, researchers add specific proteins and chemicals to the cell culture media—a nutrient-rich liquid that bathes cells to support their growth. These additions provide stem cells with instructions to form an organoid by controlling the type and position of cells within the cluster. The 3D structures that arise after months of this differentiation process are far more complex than the original clusters of stem cells. Remarkably, researchers can create organoids that resemble specific tissues in the human body, all starting with an identical ball of cells.
A new tool in the toolbox
The use of organoids is fairly new in neuroscience. Neuroscience research is traditionally conducted using a combination of animal models—mainly mice—and by studying individual neurons in plastic Petri dishes. These model systems serve as important tools but have limitations that organoids may help address.
Mice fill a special and important role in medical research. As mammals, mice share many of the same basic bodily functions as humans, including processes involved in metabolism, digestion, respiration, and ageing. Although it may come as a surprise, mice share 80 percent of their genome with humans. The genetic similarity and shared bodily processes position mice as a valuable animal model. Before entering human clinical trials, novel drugs usually undergo pre-clinical testing in mouse models to evaluate safety and efficacy. In addition, disease-specific mutations can be edited into the mouse genome to create a mouse model of a specific human disease, such as autism spectrum disorders and amyotrophic lateral sclerosis.
Live mouse models are critical for observing behavioral or psychological changes that accompany many diseases of the brain. Yet, despite their genetic similarity to humans, mice are not a perfect replica of human disease. Emily Bulger, a graduate student studying neurodevelopment in the Developmental & Stem Cell Biology Program at the University of California, San Francisco (UCSF), explains, “We have learned a lot about human development through mouse models, but at the end of the day, mice are not humans and as a result, there are developmental differences between the two species.” Beyond development, the brains of humans and mice have genetic and functional divergences throughout adulthood as well. UC Berkeley Associate Professor of Neurobiology Helen Bateup notes, “There are genes expressed in humans that aren’t expressed in mice, and some of these genes are known to cause disease.” This is not to say that animal models of disease are useless, only that caution should be taken when extrapolating results from mice to humans. The recent failures of multiple drugs in clinical trials for Alzheimer’s, a degenerative brain disease and the most common cause of dementia, have echoed a similar message: mice are sometimes poor predictors of human disease and response to therapeutics.
At times, an entire living organism is not necessary for biomedical research. For scientific questions involving a single cell type or protein, the size and complexity of a mouse can be excessive. In these instances, researchers instead study individual cells in a Petri dish, a technique termed in vitro cell culture. Cell culture involves growing cells, often isolated from human or mouse tissue, outside of the organism in a controlled environment. Cells attach to the flat surface of a Petri dish and tend to spread outwards, like a pancake. For this reason, traditional cell culture is often referred to as two-dimensional (2D) cell culture. In contrast to animal models, 2D cell culture is simple, inexpensive, and reproducible. It is often preferred when studying processes at the level of an individual cell, which is difficult to do in a whole living organism with hundreds of cell types and interconnected organ systems.
The simplicity of 2-D cell culture can speed up planning, executing, and analyzing experiments, but it also raises concern about the relevance of growing cells on flat plastic surfaces. Neurons in the human brain, for example, differ from the flat cells seen in Petri dishes: they live in a 3D environment with extensions and connections to cells and brain tissue in all directions. Sanjay Kumar, professor in the Department of Bioengineering and the Department of Chemical and Biomolecular Engineering at UC Berkeley, notes that differences in cell shape have real biological consequences on cell behavior. “Interestingly, dimensionality alone can profoundly affect cell responses to cancer therapy,” Kumar explains. His research group develops 3D cell culture platforms to investigate glioblastoma, a highly malignant type of brain cancer. Previous studies have shown that brain cancer cells grown in 3D culture display greater resistance to chemotherapy drugs compared to cells in 2D cultures, which died following treatment. As a result, the utility of 2D cell culture to predict drug efficacy and study complex cellular processes has been called into question. In response, neuroscientists and other researchers have turned to 3D cell culture.
You are now entering the third dimension
While some of the first experiments involving 3D cell culture can be traced back to the mid-1950s, the scientific community largely ignored the third dimension until the late 20th century. At the time, the prevailing view was that cell behavior (phenotype) could be explained by looking at the genes scattered throughout the genome (genotype). The “genotype drives phenotype” dogma guided how scientists investigated human disease and ultimately gave rise to the Human Genome Project, an international research project started in 1990 with the goal of identifying and mapping all of the genes of the human genome.
In the early 1990s, the Lawrence Berkeley Laboratory (LBL) was named one of three human genome centers under the United States Department of Energy. Researchers worked feverishly to sequence portions of human DNA and take part in the grand endeavor, but not everyone was convinced that the key to understanding disease could be found in the human genome alone.
While leading LBL’s Life Sciences Division, Mina Bissell, now an LBL distinguished scientist, found evidence for an additional regulator of cell behavior: the environment. Bissell noticed that when epithelial cells from mouse mammary tissue were cultured in Petri dishes, they stopped producing milk—a hallmark function of mammary tissue. Bissell was intrigued. She hypothesized that the reason the cells were misbehaving was related to their 2D cell culture environment. Bissel wondered if modifying in vitro culture to appear more similar to the mouse mammary tissue could encourage epithelial cells to secrete milk proteins once again.
Cells found within an organism, referred to as in vivo, are surrounded by a dense network of non-cellular components known as the extracellular matrix. To mimic the extracellular matrix in a Petri dish, Bissell cultured mammary epithelial cells within two 3D extracellular matrices that resemble mouse breast tissue: rat tail tendon and mouse sarcoma tumor. Compared to epithelial cells grown on plastic Petri dishes, the cells in contact with the 3D matrix formed 3D clusters with internal structures resembling mammary glands. Even more, they secreted milk proteins!
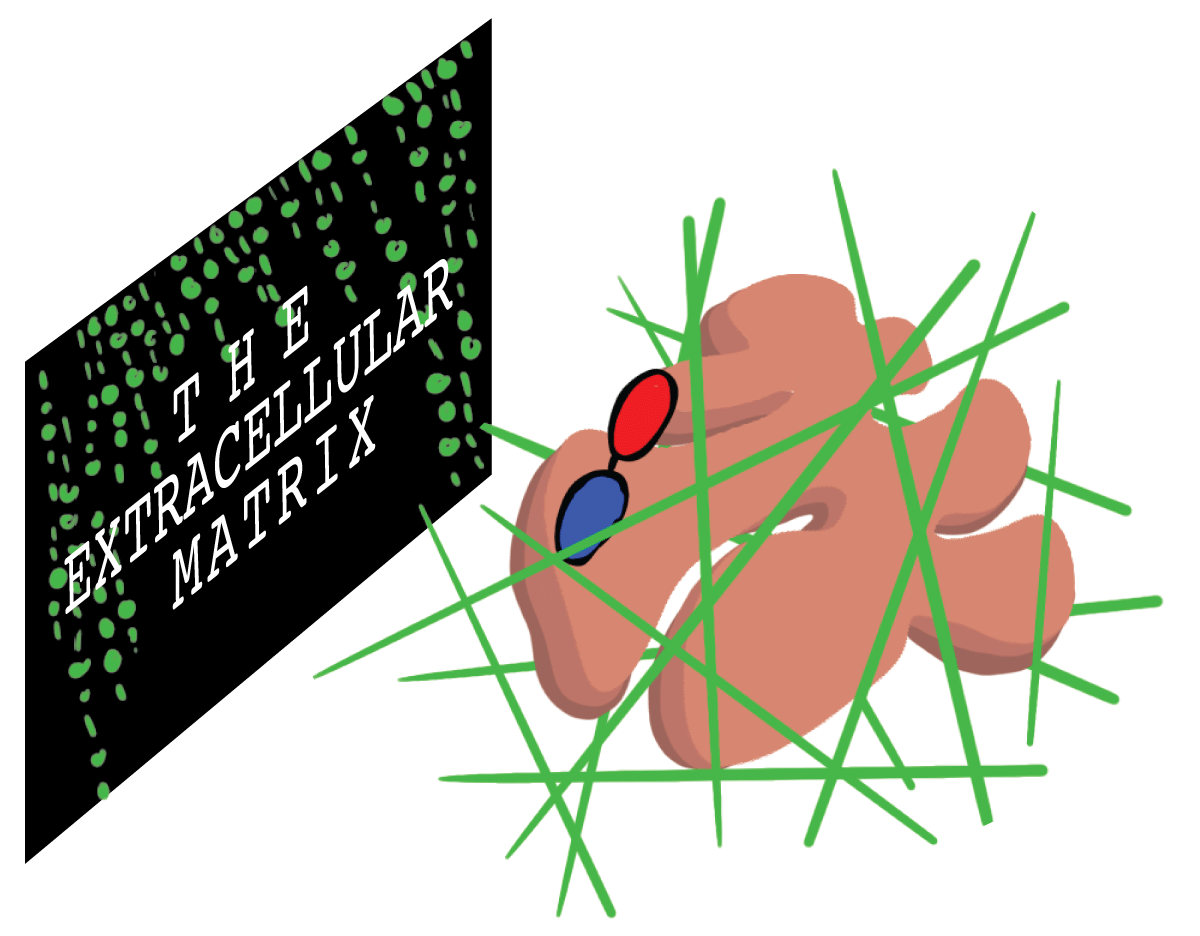
The findings were extraordinary. Bissell demonstrated that when cells are cultured in a 3D environment similar to their in vivo environment, their structures and functions are comparable to those of cells in a living organism. But the biological community was not convinced. In a 2002 interview with the Palo Alto-based pharmaceutical company Incyte, Bissell recounts, “In the United States, scientists basically didn't take the idea seriously.” However, in the nearly 35 years since Bissell’s early experiments with mammary tissue, hundreds of additional studies and publications have proved Bissell’s original theories were correct, and biology’s third dimension finally reached center stage.
With a new appreciation for 3D cell culture, scientists began growing stem cells in three dimensions to form the first organoids, converting a 3D balls of identical stem cells into miniaturized organs that resemble tissue.
Getting a glimpse of brain development
Although they haven’t yet found their way into every biomedical research lab, brain organoids are an indispensable tool for studying many diseases, including neurodevelopmental disorders. Neurodevelopment is the process by which the brain and spinal cord, collectively termed the central nervous system (CNS), grow and develop. Advances in DNA sequencing methods have revealed that a large proportion of neurodevelopmental disorders have an underlying genetic cause. But as Bissell demonstrated, identifying the diseased gene is only part of the puzzle, so researchers have begun using organoids to model CNS development in 3D environments, similar to how the brain and spinal cord form in humans.
The Bateup lab at UC Berkeley studies tuberous sclerosis complex (TSC), a developmental disorder that causes the formation of cortical tubers—regions of abnormal cells—in the brain’s outermost layer during early development. Cortical tubers contain dense masses of dysfunctional cells that disrupt the brain’s highly organized and interconnected cellular network. Patients with TSC often have neurological and psychiatric complications, including cognitive impairment and behavioral disorders. Bateup explains, “One of the main concerns in patients with TSC is the seizures because they can start very early and become intractable.” While it is known that TSC is caused by mutations in the TSC1 or TSC2 gene, it remains unknown how TSC mutations and cortical tuber formation impact neural development and function.
Investigating neurodevelopmental disorders leads to a great deal of technical and ethical challenges. Brain tissue donated to biomedical research is usually from patients that have undergone neurosurgery. For researchers studying early brain development, these tissue samples are often too mature, so mice and other animal models are advantageous, since they can be monitored during both early brain development and adulthood. Mouse models have been a powerful research tool in the Bateup lab, especially for modeling the behavioral and psychiatric conditions associated with TSC. However, one important aspect of TSC, the presence of cortical tubers, is not consistently observed in mouse models of the disease. While it is unclear why TSC mice don’t reliably form cortical tubers, Bateup explains that “as a result, we don’t really understand well where these early brain developmental alterations come from in patients with TSC.” What they really needed was a human system that could model early developmental stages of TSC—cue the human brain organoids.
In collaboration with Associate Professor Dirk Hockemeyer’s lab in the Department of Molecular & Cell Biology at UC Berkeley, the Bateup lab uses CRISPR-Cas9 gene editing technology to establish neural organoids containing TSC1/2 mutations. The researchers start by forming clusters of either normal or gene-edited stem cells, which ultimately become either healthy or TSC organoids, respectively. They then add a cocktail of molecules to the media surrounding the clusters, which instructs the stem cells to convert into neurons and other brain cells. After a couple of months, the composition and organization of cells within the 3D structures resemble that of a fetal human brain.
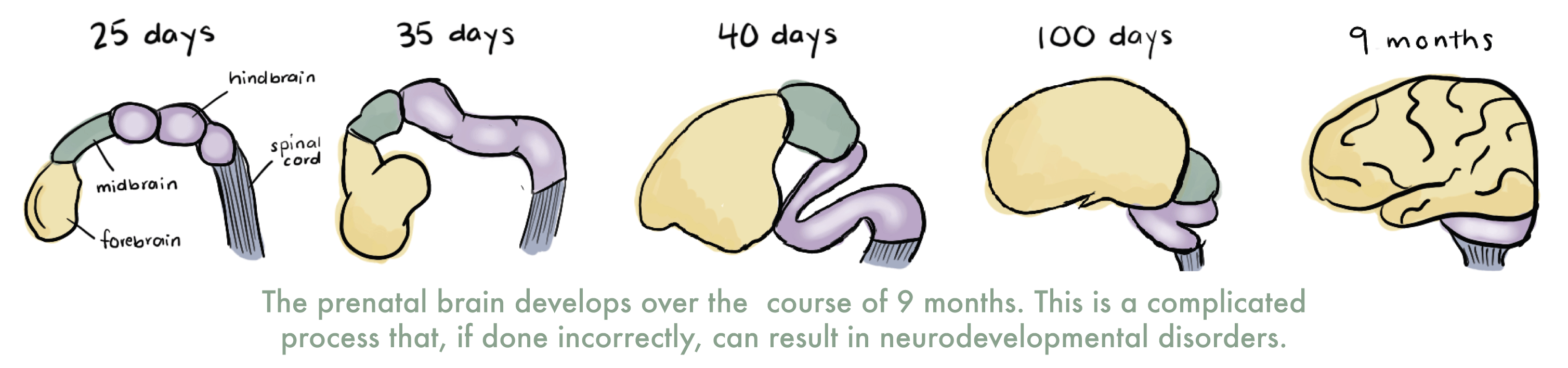
Interestingly, the development of brain organoids follows roughly the same time course of that of the human brain. Bateup explains, “[Organoids] continue to develop over time and they develop in roughly real time.” Until recently, it was unclear whether brain organoids could mature beyond mid- to late-fetal stages. However, in February 2021, researchers at Stanford University and University of California, Los Angeles, cultured 600-day-old brain organoids and demonstrated that cells within the organoids experience several of the same developmental milestones as human brains. Bateup adds, “If you culture [organoids] for nine months or more, they spontaneously start switching to a more postnatal-like stage, which is fascinating.”
While the relatively slow and time-consuming process of mature organoid formation may be cumbersome for researchers seeking to study late developmental processes, the timescale is less of an issue for those interested in modeling early brain developmental processes, such as cortical tuber formation. “If you start an organoid and culture it for six months, that’s roughly equivalent to a six-month gestational age fetus,” Bateup says.
In their recent study, the Bateup lab cultured normal and TSC brain organoids for eight months to identify the approximate timeline of cortical tubers formation during brain development in patients with TSC. The first two months of TSC organoid development mirrored that of the healthy organoids, but by day 100, the organoids began to diverge. TSC organoids contained neurons and other brain cells that were large and oddly shaped—two distinguishing features of cells within cortical tubers. Next, the Bateup lab tested the ability of rapamycin, a drug for patients with TSC, to inhibit or reverse cortical tuber formation in TSC organoids, and they identified a critical developmental period (days 25-43) in which rapamycin treatment suppressed tuber formation in organoids. Outside of this critical window, rapamycin did not reverse cortical tuber formation and actually led to additional developmental abnormalities in TSC organoids.
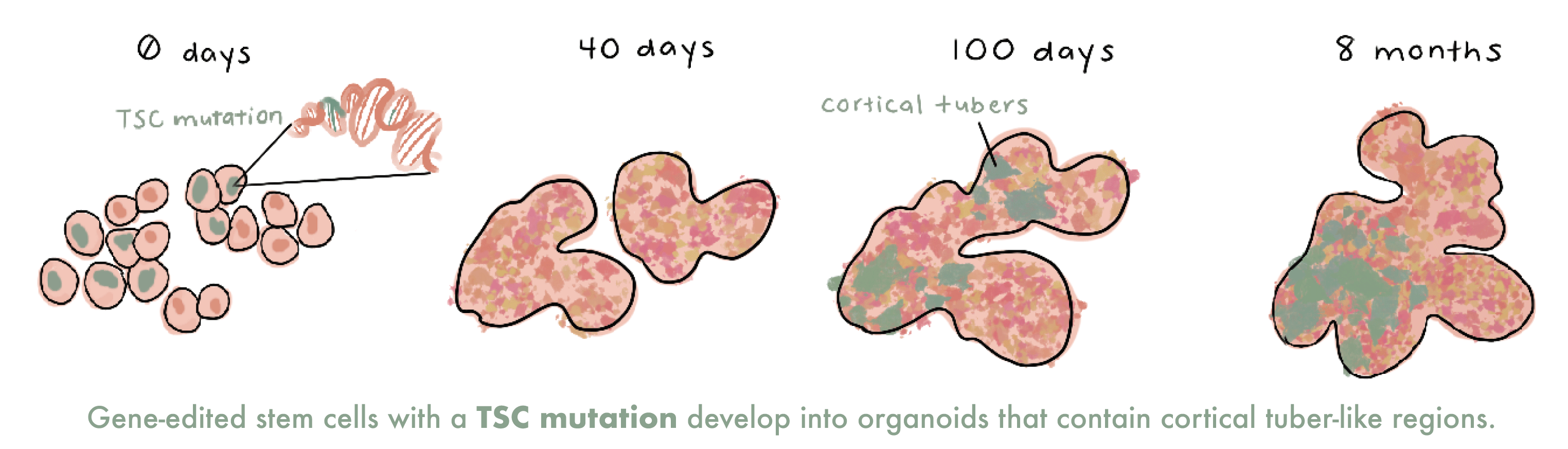
Their finding that rapamycin treatment early in organoid development suppresses cortical tuber formation has important clinical implications when designing treatment strategies for patients with TSC. “Organoids open up a lot more avenues of investigation about the basics of human development that we can’t tackle any other way,” Bateup says. Her team is excited to use their organoid model to perform additional testing of therapeutics for TSC and identify optimal treatment windows to have the greatest impact on a patient’s quality of life while minimizing potential side effects.
Repairing neural connections after spinal cord injury
On the other side of the Bay, researchers at Gladstone Institutes, a UCSF-affiliated research center, are using neural organoids for a different purpose: to study how neuronal connections form and survive in the human spinal cord.
The spinal cord is a column of neural tissue that runs from the base of the skull down the center of the back. It is the highway for communication between the body and the brain, but it also acts independently from the brain as the reflex center. A reflex is an unconscious response, or an automatic action that does not require brain processing. A simple and familiar example of a reflex is the knee jerk—when a doctor taps on a spot just below your kneecap with a rubber hammer and that leg kicks out. As the hammer touches the kneecap, sensory neurons within the muscles of the knee joint are activated and send an impulse to interneurons in the spinal cord. An interneuron is a type of neuron that receives and transmits information between other types of neurons. After receiving the first signal, the interneurons send the impulse back to the knee, triggering a muscle contraction. As it turns out, kicking your doctor requires zero brain power.
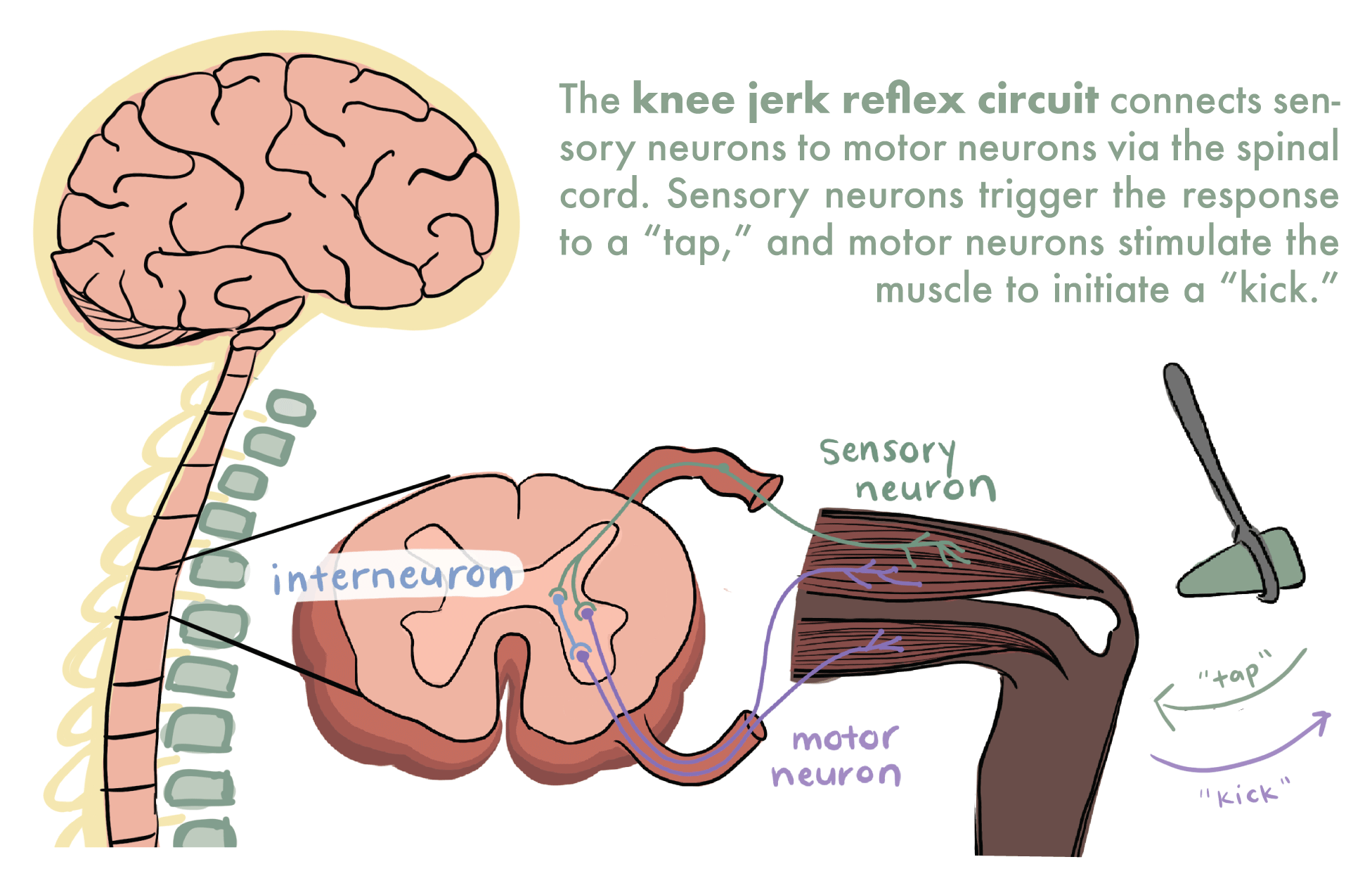
The knee jerk reflex is an example of a neural circuit, which is an organized network of neurons functioning together. Lana Zholudeva, a postdoc in Todd McDevitt’s lab at Gladstone Institutes explains: “In a complex central nervous system, there are individual components [neurons] that are important, but these cells must sense each other, talk to each other, and ultimately build a circuit in order to quickly perform their functions.”
The McDevitt lab uses organoids to investigate how neuronal connections form and survive in the spinal cord. During organoid fabrication, Zholudeva instructs the 3D stem cell clusters to become interneurons, the neurons that send and receive information. The interneuron organoids provide Zholudeva with a valuable tool to study human interneuron circuitry and function in a healthy spinal cord, as well as in diseased conditions, such as after spinal cord injury.
Although protected behind the backbone, the spinal cord is not completely unsusceptible to injury. Spinal cord injury can occur in a variety of ways, including physical trauma to neural tissue. Neural connections can also break down as a result of disease and cause abnormal movement such as uncontrollable tremors, which are symptoms of Parkinson’s disease. The spinal cord rarely repairs itself after damage or injury, but many researchers, including Zholudeva, are increasingly optimistic that they will one day find a way to spark the repair of the spinal cord’s indispensable cells and circuits.
Converting stem cells into interneurons was a daunting task, since typical neuronal differentiation protocols mostly produce a different type of neuron, the motor neuron. However, in a previous publication, the McDevitt lab identified a cocktail of molecules that coaxed human stem cells into becoming functional interneurons for the first time. They recently applied their 2D interneuron differentiation technique to 3D stem cell cultures to investigate interneuron development and function in human neural organoids. They monitored interneuron maturation and function by recording electrical activity. After 50 days in culture, organoids began to show synchronous electrical signals, indicating interneuron maturation.
Studying interneurons in neural organoids will provide a deeper understanding of interneuron behavior in the human spinal cord, and the McDevitt lab hopes their discoveries will guide the development of novel treatments for spinal cord injury and disease. They are specifically interested in replacing damaged cells of the spinal cord with healthy interneurons. Zholudeva explains, “If we put the cells into a broken system, are they smart enough to figure out how to wire appropriately?” Zholudeva notes that forming connections with neighboring cells is only one part of an interneuron’s responsibility. The next step is ensuring that the connections function properly. In the knee jerk example, a functional interneuron needs to receive the initial signal (tap!) and send the secondary signal (kick!).
Replacing the damaged cells with functional interneurons could improve a patient’s sensor perception and movement, but successful cell integration is not guaranteed. “Although we may be figuring out how to engineer different types of cells that may benefit repair and tailoring them to the needs of the individual we want to treat, we are not telling them who to connect to yet,” Zholudeva explains. As we know from Bissel’s 3D mammary tissue experiments, the cellular environment is key. A healthy neuron transplanted in a diseased spinal cord is in a vulnerable position. The long-term survival and function of interneurons after transplantation is an exciting area of investigation for the McDevitt lab, and Zholudeva believes that the future of interneuron cell replacement is bright.
Can consciousness be created from scratch?
The idea of scientists growing mini-brains invokes a frightening mental picture, but the reality of the research is still quite far from a full-fledged human brain in a dish. Bateup explains that while “no other system really captures the early development of the human brain the way that organoids do,” these cell clusters are still far from being human. A small ball of cells grown in a dish may serve as a useful model for specific aspects of brain development and function, but they do not represent the entire human brain. Zholudeva admits, “We have to appreciate the underlying biology of what it takes to develop a human being and a human CNS. In a dish, we are not replicating any of that.”
Present day organoids do not contain all of the brain-specific cell types required for human consciousness and those that are included don’t survive long enough to reach a mature, adult-like stage. Bateup adds, “They’re not mini-brains at all. The organization within them is not even close to the beautiful organization of the evolved human brain.” Even as researchers learn to include additional brain-specific cell types and grow organoids in more realistic conditions, it is possible that organoids will never be able to truly replicate the brain’s anatomical structure and complexity. Zholudeva agrees, “I just don’t quite see it as possible … not within my own lifetime.”
Some scientists, however, are apprehensive about further developments in brain organoid technology. In 2019, a research lab led by Alysson Muotri at the University of California, San Diego, reported the creation of brain organoids with electrical activity resembling that seen in an immature human brain. Muotri’s group used microelectrodes to measure tiny changes in electrical activity and demonstrated that over the course of a year, the organoids’ activity transitioned from scattered, single frequency activity to regular, coordinated waves of electrical activity. Brain-wide coordinated electrical activity is one feature of a conscious brain, and as a result, this study has sparked a lively conversation about the potential ethical implications brain organoid research.
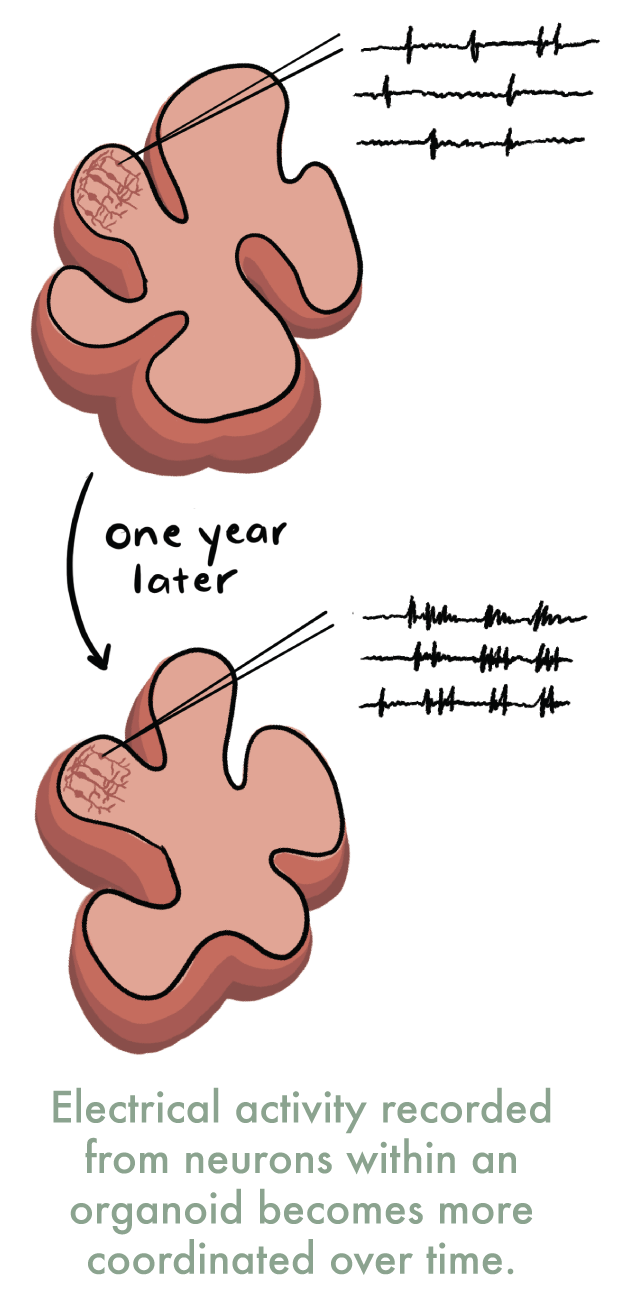
Questions around brain organoid consciousness have highlighted another enigma—how is consciousness defined and measured? Besides recording brain-wide electrical activity, an additional clinical test for consciousness is performed by assessing a patient’s response to light stimuli. Standard medical exams such as these are difficult to apply to 3D cell cultures—last time we checked, organoids can’t blink. “I think this is where philosophy should meet science, and philosophers alongside bioethicists can shed some light on the guidelines and really educate the scientists in terms of what it is to be human,” suggests Zholudeva.
Brain organoids and beyond
While the question of organoid sentience is still up for debate, most scientists can agree that organoids are important tools for biomedical research. Ana Carneiro, a graduate student in the UC Berkeley’s Department of Chemical and Biomolecular Engineering who works in Dave Schaffer’s lab explains, “Without actually going into a human subject, we can use organoids to further understand the fundamentals of the human body.” The Schaffer lab uses brain organoids to develop and improve viral-based gene delivery techniques. “We hope to identify novel viral variants that can successfully target specific cell types within the human brain,” Carneiro says.
In addition to the brain, researchers at UC Berkeley are also creating organoids of other organs, including intestinal organoids in the laboratory of Professor Russel Vance in the Department of Molecular & Cell Biology, or cardiac organoids in Professor Kevin Healy’s lab in the Departments of Bioengineering and Materials Science & Engineering. As stem cell differentiation protocols improve, the complexity of organoids will follow. One can envision a day when tiny balls of cells can mimic every tissue in the human body.
----- Erin Akins is a graduate student in bioengineering.
This article is part of the Spring 2021 issue.