Cobamide co-op
How gut bacteria share essential vitamins
Beyond the quarantine hobby, bakers have played critical roles in feeding communities for centuries by making bread, not just for themselves but for others as well. A recent discovery by UC Berkeley researchers shows that a human gut bacterium could be quite the “nutrient baker” itself!
The human gut is teeming with bacteria that form a community called the gut flora. Like many bacterial communities in nature, the gut flora features different species of microorganisms that all share the same ecosystem. Associate Professor of Plant and Microbial Biology Michiko Taga runs a lab that studies these small-scale ecosystems at the molecular level: “We’re aiming to understand how microbial communities work, which is a difficult question."
Bacteria in microbial communities interact with each other by sharing nutrients, but the specific mechanisms of how they do so remain unknown. For example, the Taga group focuses on how bacteria share cobamides, a family of nutrients including Vitamin B12 that are required in several metabolic pathways. While the majority of bacteria use cobamides in their metabolism, only a subset of microorganisms can produce them. Taga explains that “we know that there are cobamide producers, and, somehow, cobamides are taken up by the bacteria that require cobamides in the community. But we don’t know how that happens.”
Many different types of cobamides exist, and sharing nutrients within the gut is especially complex because bacteria in the same community may all require different cobamide types. Dr. Kenny Mok, senior project scientist in the Taga group, explains, “Our lab is interested in this idea of cobamide specificity, where we’ve noticed there's all these different forms of cobamides, but different bacteria only make or use specific forms.” To better understand how cobamide specificity affects which microbes grow in the gut, Mok cultivated different species of gut bacteria in flasks containing one of seven different types of cobamides. If a bacterial species preferred one type of cobamide, it should grow better in environments containing that cobamide than in environments containing other versions. However, that was not the case for one species: Akkermansia muciniphila. “When I tested the growth [of A. muciniphila.] with different cobamides, I noticed it was basically identical,” explains Mok.
This result was surprising. A. muciniphila is known to require a very specific type of cobamide called pseudocobalamin for its metabolism, but is unable to produce pseudocobalamin on its own. Why did A. muciniphila exhibit no apparent specificity to cobamides?
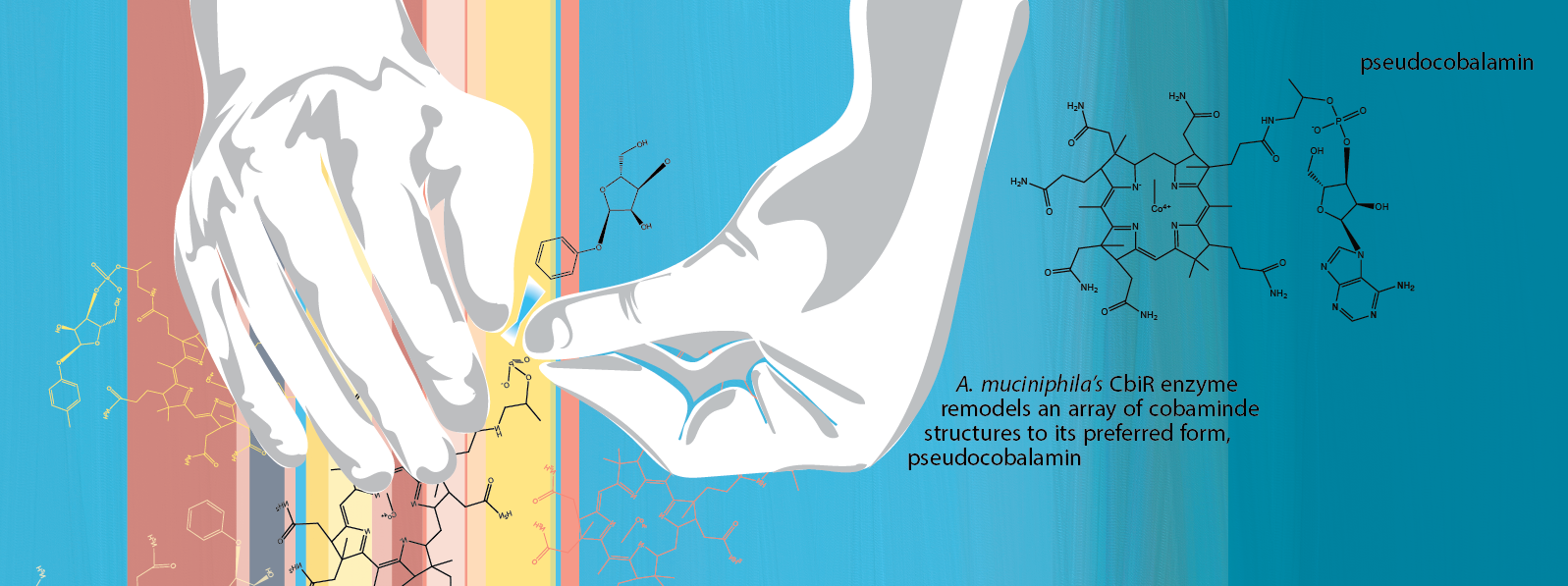
One possible explanation was that A. muciniphila was transforming all the different types of cobamides it was supplied with into the one form it could use. “If you convert [all cobamide types] to one form, the growth response should be more or less the same,” Mok explains. Indeed, when the researchers looked at which cobamides remained in the culture, the originally added ones were gone--in their place, they found pseudocobalamin. This transformation suggests A. muciniphila was successfully converting the different types of cobamides available in the environment into the specific kind it requires.
Upon further analysis, the researchers discovered that A. muciniphila was using a protein called CbiR to chemically transform available cobamides in its environment into the type it could use, a process called cobamide remodeling. Taga explains, “We don’t know yet if A. muciniphila remodels cobamides in the gut [as it does in an experimental flask], but if it is, it could shape the cobamide composition of the gut, and that could influence which other microbes are able to persist in the gut environment.” A. muciniphila may act as a sort of microscopic community baker, transforming nutrients into usable forms for itself and other bacteria.
The Taga group’s discovery of CbiR provides important insight into how members of our gut flora share nutrients. Genetic analyses reveal proteins like CbiR are found in more than 200 other bacterial groups, and not just in the gut. Further research is needed to understand the roles of these CbiR-like enzymes, but cobamide remodeling may be more widespread than previously thought! If so, “nutrient bakers” like A. muciniphila, who modulate the type of nutrients available in their microbial communities, may be a crucial piece of the puzzle of how and why bacteria share nutrients!
----- Héctor L. Torres Vera is a graduate student in molecular and cell biology.
This article is part of the Spring 2021 issue.