Jesse Gelsinger died in November 1999 after receiving experimental treatment for orthinine transcarbamylase deficiency, an obscure disease which prevents the breakdown of metabolic waste products. His death heralded a monumental shift in the aspirations and hopes of researchers and physicians around the world. Just days earlier, gene therapy—modifying the DNA of a patient—was hyped as a miraculous cure for several seemingly incurable and deadly diseases, ranging from Huntington’s to cancer. However, the field was abruptly quieted following the highly publicized deaths of several patients enrolled in gene therapy clinical trials, including Gelsinger.
Gelsinger’s death wasn’t caused by the artificial gene injected into his body, but instead by a fatal allergic reaction to the type of virus, known as lentivirus, that was used to carry the gene from the injection site into his cells. Appropriate delivery of DNA cargo through a carrier ship that navigates to the correct destination has been regarded as one of the largest obstacles for gene therapy. Nowadays, thanks to decades of innovations, scientists are finally successfully changing mutated genes in sick patients and generating treatments to countless heritable diseases.
What is gene therapy?
Gene therapy introduces new genetic material into a patient’s DNA to cure disease. DNA contains instructions to create proteins that perform essential functions in the body, and genetic diseases are caused by mistakes or errors in the DNA that lead to missing or dysfunctional proteins. As early researchers discovered this connection, they hoped to create artificial genes or DNA that encoded the dysfunctional protein correctly. Once the artificial DNA was delivered into patients’ cells, the cells would have the correct manual for making the appropriate protein.
Yet gene replacement therapy was not without its technical hurdles. Integrating an extra gene into patient DNA, where it was not originally intended to fit, often had unexpected consequences on the surrounding DNA, depending on the size of the artificial gene. In some cases, adjacent genes, or even the integrated gene itself, would suddenly generate more or less proteins than expected. The situation was akin to a young adult who invited their family to live with them. The upheaval in their lifestyle might be minimal if only a single sibling arrived, but would likely increase as more of their extended family decided to move in.
However, in 2012, UC Berkeley chemistry and molecular & cell biology professor Jennifer Doudna found a way to modify DNA that circumvented this obstacle, earning her the Nobel Prize in Chemistry in 2020. She used a new technique called CRISPR/Cas9 genome editing. Cas9 is a protein originally discovered in bacteria that can find a specific DNA sequence and cut it, allowing researchers to more easily delete or edit a specific gene. Cas9 is so specific because it relies on a fragment of RNA, a closely related, single-stranded cousin to double-stranded DNA. This fragment of RNA is aptly named a guide RNA, because it guides Cas9 to its cut site by matching and intertwining itself with a complementary piece of DNA targeted for editing. By making precise edits straight into a mutated gene, researchers avoid introducing an entire new copy of the gene.
Whether or not doctors use conventional or CRISPR/Cas9-based methods to treat genetic illnesses in the future, all the materials for treatment must be delivered to the correct cells within a patient. The Innovative Genomic Institute (IGI), a partnership between UC Berkeley and UC San Francisco aiming to cure disease using gene therapy, has pioneered several solutions to this problem. In particular, researchers affiliated with IGI have made strides by approaching the delivery of gene therapy in two distinct ways: ex vivo and in vivo.
Curing sickle cell anemia using ex vivo gene therapy
In late 2019, Victoria Gray became the first human to be successfully treated using CRISPR/Cas9-mediated gene therapy. She suffered from a devastating disorder predominantly affecting those of African descent called sickle cell anemia. Her success story was built on the foundation of years of academic research, particularly a 2016 study from former IGI and UC Berkeley Professor Jacob Corn’s lab. They reported on how to cure mice of sickle cell anemia using an ex vivo gene therapy strategy, which relies on removing diseased cells from a patient, treating them outside their body, and then reintroducing the treated cells back into the patient. This same ex vivo strategy was ultimately used to cure Gray.
IGI scientific director and UC Berkeley Molecular & Cell Biology Professor Fyodor Urnov describes the first major motivation for targeting sickle cell anemia: opportunity. “When a publicly held company approaches a situation like this, they need to understand how will this be profitable, so you need some number of people,” Urnov explains. “Sickle has 100,000 people [in the United States].” As a comparison, around 80,000 people are diagnosed with Parkinson’s disease in the United States each year. Urnov continues, “The life expectancy is 40-42 [years], so the unmet medical need is reasonably high.”
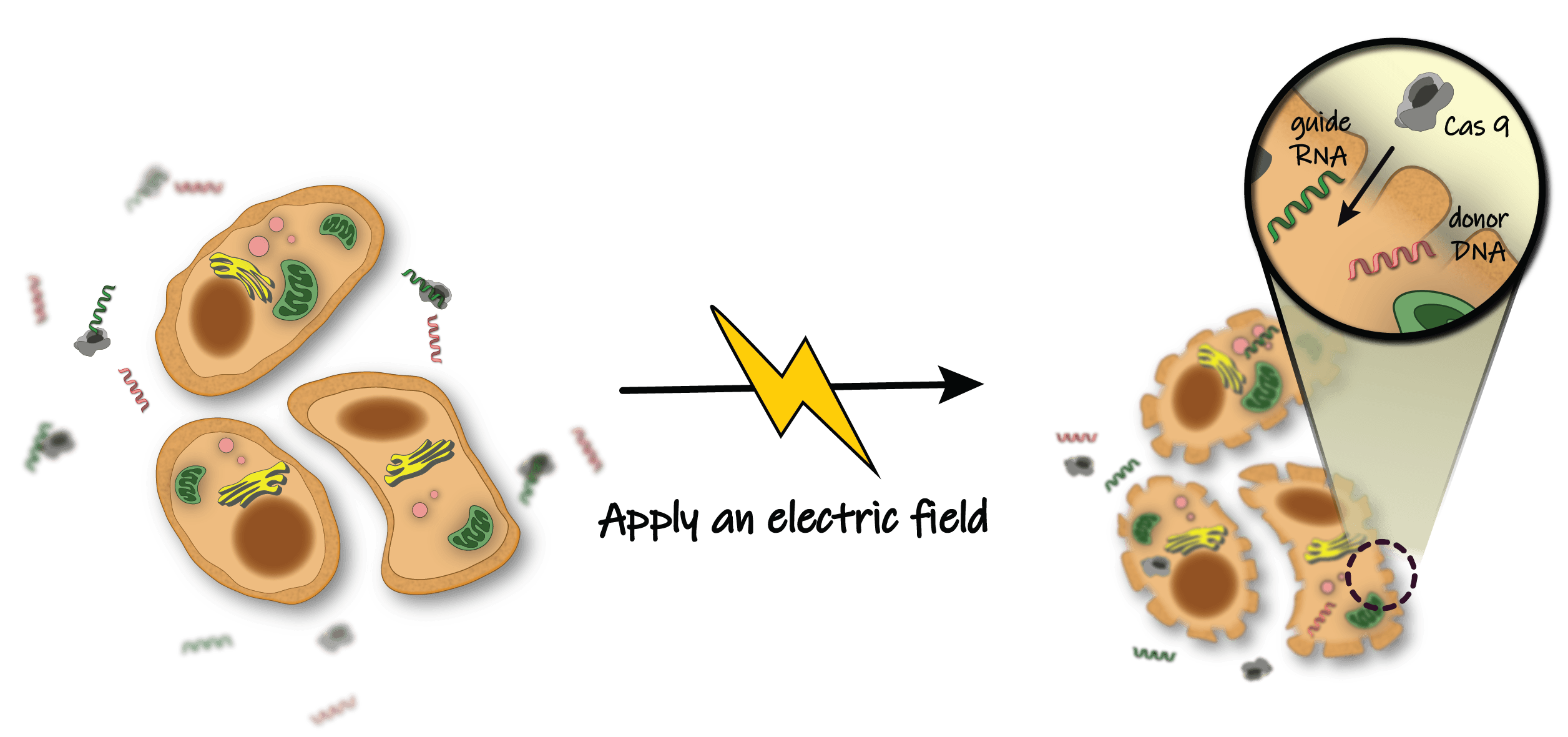 The membrane of a cell acts as a barrier blocking the entry of most particles. Application of an electric field creates 'holes' in the membrane. These holes are large enough to allow gene editing components (i.e. CRISPR/Cas9) to easily cross the membrane barrier.
The membrane of a cell acts as a barrier blocking the entry of most particles. Application of an electric field creates 'holes' in the membrane. These holes are large enough to allow gene editing components (i.e. CRISPR/Cas9) to easily cross the membrane barrier.
Another huge appeal is that sickle cell anemia is especially amenable to ex vivo delivery. Sickle cell anemia is a blood disorder that transforms healthy blood cells into sickle moon-shaped cells that clog arteries and cannot deliver as much oxygen throughout the body. Researchers are able to siphon and collect the sickened blood cells from the body. Then, they can treat the cells by zapping them with an electric shock. The shock causes the cells to open temporary holes in their cell walls, allowing the CRISPR/Cas9 reagents to move inside and perform the appropriate edits. Scientists observe which of the cells had their DNA correctly edited by Cas9 and transfer only those corrected blood cells back into the patient where they can now function correctly.
The idea of removing cells from a patient, treating them while they sit in a petri dish in a lab, and then returning them back into the patient—ex vivo delivery—contrasts with in vivo delivery, where DNA is transferred into cells while they remain in the patient’s body. “In practical terms, ex vivo editing is easier than in vivo,” Urnov says. Since cells are treated outside the body during ex vivo gene therapy, researchers can check that the cells are properly corrected and functioning appropriately before returning them to the patient. Additionally, in vivo delivery requires the gene therapy reagents to successfully travel through multiple organ systems in the human body before arriving at the correct tissues. When delivering gene therapy reagents to cells in a petri dish, the chances of successful delivery are much higher.
Despite its advantages, ex vivo delivery restricts treatment to very few diseases. Doctors can remove unhealthy blood cells and then inject corrected blood cells into a patient, but they cannot so easily cut away a piece of a patient’s damaged brain and replace it with new brain cells. As a result, researchers must pivot to in vivo gene therapy to cure most genetic illness.
Changing the destination in in vivo gene therapy
Although numerous viral-mediated gene therapy clinical trials failed throughout the 1990s and early 2000s, including the lentivirus that killed Gelsinger, researchers have continued to hijack viruses to deliver gene therapies. They push forward despite the historical failures because viruses have mastered the art of injecting foreign DNA into human cells. In fact, around eight percent of the human genome is estimated to be made of sequences that originated from viruses. The most promising viral carrier is the adeno-associated virus (AAV), a small, naturally occurring virus that infects humans and other primate species but is not currently known to cause disease.
IGI and UC Berkeley Professor of Bioengineering Dave Schaffer’s lab specializes in developing novel AAVs to target specific organs in the body. Viruses that are better at traveling to particular tissues both increase the chance a tissue’s diseased DNA will be changed and reduce the chance of any sort of catastrophic allergic reaction. Although these viruses exist in nature, most natural AAVs don’t target specific organs. Schaffer explains, “the field has been compensating for that by really amping up the [viral] dose, and still a tiny fraction of the natural AAV makes it to the right tissue. As a result of the increased dosage, however, the AAV rises above the immune system’s radar, which can lead to toxicity.” By developing more efficient AAVs, doctors can lower the dosage of virus delivered into the human tissue to avoid detection by the immune system and decrease the chance of a negative reaction.
The key to changing AAVs lies in their structure. The AAV consists of a shell, called a capsid, which contains DNA. Each of the twelve viruses, AAV1-12, that naturally infect primates has a unique capsid. Normally, the viral DNA contains instructions on how to replicate the capsid so that when it is injected into a cell, the cell is able to make more viral particles. In the case of gene therapy, the AAV DNA is replaced with instructions on how to make the dysfunctional or missing protein that is causing the disease.
While DNA is a critical component of gene therapy, the capsid is what most interests the Schaffer lab because its composition dictates the AAV’s cellular target. Changes to the capsid surface increase the ability of viruses to find specific cell types, thus dictating where the AAV goes, how efficiently it travels, and the probability that it will cause an unintended allergic response.
The concept of altering the viral capsid’s structure to control its delivery in the body started in the 1990s. But despite the immense potential of capsid engineering, scientists were unable to figure out how to develop new AAVs that would only travel to specific tissue types. Researchers would redesign specific parts of the AAV capsid based on their understanding of virus biology to change where it traveled, a technique called rational design or engineering. However, the capsid was so complex that researchers were unable to use rational design to successfully determine all the changes they should make. They would inevitably steal away any ability of the virus to deliver gene editing components.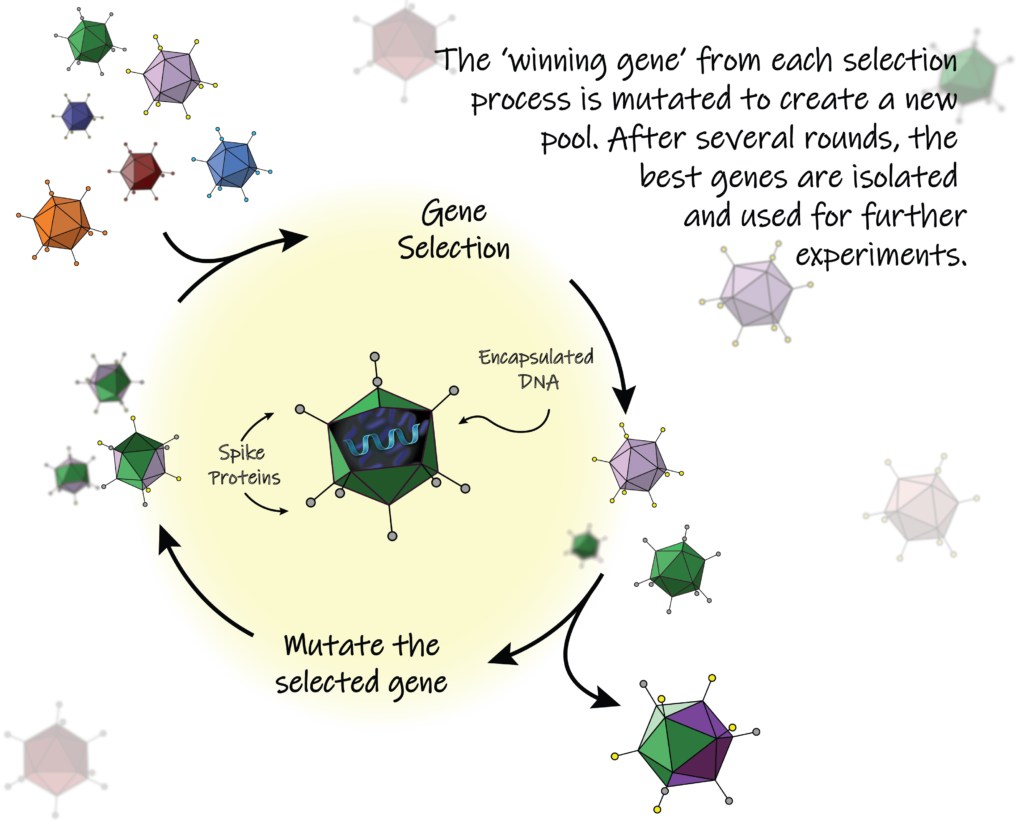
Instead, Schaffer decided to use a different strategy to re-engineer the AAV: directed evolution. Directed evolution was developed by UC Berkeley alum Frances Arnold, who was awarded the Nobel Prize in Chemistry in 2018 for pioneering the use of this technique to engineer enzymes. The strategy of directed evolution emulates natural evolution by creating a large pool of randomly mutated genes before selecting the genes that best survive a particular task. This process is repeated several times by mutating the winner gene from the selection process into a new, diverse pool. Since directed evolution is performed in a controlled lab, results occur more quickly than they do in nature, where creatures evolve over millions of years. “I began to apply directed evolution towards viruses like AAV for gene therapy applications in 1999, and it’s one of those ideas that ended up working even better than I had hoped,” Schaffer says. Specifically, his lab would randomly mutate—or perform random mutagenesis on—the capsids of AAVs and then track which AAVs traveled to the tissue where researchers wanted to deliver their therapy.
Directed evolution is ideal for engineering AAVs because, contrary to rational design, it can test many capsids at a time. As Schaffer explains, “With rational engineering, you’re basically making one mutation at a time—taking one shot on goal at a time. By doing random mutagenesis, we now have a gene pool over a billion AAV variants. So, we’re not taking shots on goal one at a time. We’re taking a billion shots…all at once.”
Those shots on goal have not gone to waste. Fifteen years of hard work have resulted in delivering a virus into the retina to combat several different retinal disorders. Industry connections and collaborations with the Schaffer lab have led to four ongoing gene therapy clinical trials using engineered AAVs in the retina to combat blindness and other eye disorders. The transformation of academic work on wily viruses to direct improvements in human health with clinically tractable treatments that may soon cure patient populations is exactly what Schaffer has always hoped to achieve in his lab. “The fact that we have gotten technology from our lab at Berkeley into multiple human clinical trials is something I’m very proud of,” Schaffer says.
Replacing viruses with gold
Although AAV-mediated gene delivery has had numerous successes, some researchers are pivoting away from using viruses altogether. Instead, they are overcoming the lingering safety concerns in viruses by creating synthetic carriers to deliver traditional and CRISPR/Cas9-based therapies. One of the most promising non-viral carriers to deliver CRISPR/Cas9 into cells is called CRISPR-Gold, invented in the labs of UC Berkeley Bioengineering Professors Niren Murthy and Irina Conboy. Their work has since been catapulted into a start-up called GenEdit, founded by Kunwoo Lee, the graduate student in Murthy’s lab who spearheaded CRISPR-Gold.
CRISPR-Gold revolutionized CRISPR/Cas9 delivery by showing that it was possible to deliver additional gene editing material that would allow CRISPR/Cas9 to not just destroy, or knock out, a dysfunctional gene, but to correct a dysfunctional gene. The difference between knocking out and fixing is similar to the choice between deleting an entire misspelled word, such as “chocalate”, or replacing the misspelled word with the correctly-spelled word “chocolate.” While the original only requires Cas9 protein and a guide RNA to tell Cas9 which word to cut out, the latter requires an additional piece of material, called donor DNA, that tells the cell the correct version of the word that needs to be replaced. While some disorders result from the chaotic behavior of a mutated and misbehaving protein, other disorders result from the absence of a protein that is not present in the body. Although knocking out a gene may cure patients that belong to the first category of disease, they cannot treat patients belonging to the second category. Rather, the absence of a functional protein can only be fixed by inserting a correct version of the gene encoding the protein.
Delivering an additional piece of donor DNA along with the rest of the CRISPR/Cas9 was not a trivial task. As Murthy explains, “We focused on a very specific type of problem, which was not just the delivery of the CRISPR protein but also the delivery of the donor DNA. What Kunwoo came up with is he figured out a way of being able to make a nanoparticle that could assemble the Cas9 protein, the guide RNA, and the donor DNA.” Specifically, Kunwoo found that gold had a natural affinity to DNA which could be combined with other CRISPR/Cas9 components and an additional molecule that helped the whole CRISPR-Gold complex invade cells.
While gold provided an important starting point for delivering DNA along with Cas9, it also created a problem. Gold is not biocompatible, meaning it can accumulate in human tissue and cause people to become sick. However, GenEdit has managed to replace the gold with a synthetic molecule made of repeating molecular chains, called a polymer. This task was not straightforward. “For GenEdit to come up with something that works as well as CRISPR-Gold without the gold, they might have had to synthesize thousands of polymers and invested several million dollars,” Murthy says.
Despite these difficulties, investing in non-viral delivery methods such as CRISPR-Gold remains important due to the potential increased safety. AAVs exist naturally in the world, and in people with prior exposure to a specific AAV, there is the chance they will have a harmful immune response, similar to Gelsinger’s fatal reaction to lentivirus. Patients are less likely to have been exposed to synthetic nanotechnology, meaning they are less likely to have any sort of allergic reaction.
Most importantly, non-viral delivery can better avoid accidental DNA damage. A couple years after Gelsinger died, another clinical trial in Europe cured eighteen children of severe combined immunodeficiency (SCID) using gene therapy. SCID is better known as bubble boy disease, because if patients are not kept in perfectly sterile environments they will die from a simple cold or infection due to a dysfunctional immune system. While gene therapy cured these children of SCID, following treatment, four of the children developed leukemia, and one of them died from it. The retrovirus used to deliver the gene therapy had randomly inserted some of the DNA in the wrong place, interrupting a part of the children’s genome responsible for preventing cancer.
Unintended manipulation of DNA, similar to what occurred with the SCID cases, is called an off-target effect. While CRISPR/Cas9 gene editing tends to cut DNA more specifically than other viral-based gene editing tools, researchers still worry about Cas9 cutting in the wrong place, especially if it stays around in cells too long. Murthy describes the dilemma: “Gene editing is permanent. Once you corrected a mutation, you don’t need the Cas9 protein hanging around afterwards. In fact, having it expressed for long periods of time just increases the frequency of off-target DNA damage, so in the case of gene editing, there are some very compelling reasons to do non-viral gene editing.” Viral-based delivery methods, such as AAV, contain a DNA sequence that results in the continuous production of new Cas9, and AAV lingers in some cells for around five years. In comparison, CRISPR-Gold and other non-viral methods do not contain DNA sequences to produce Cas9 but rather add pre-formed Cas9 protein into their carriers. Cas9 is quickly degraded once it is released from its carriers, with half of it being destroyed after six to twelve hours.
While potential benefits abound, scientists acknowledge the long battle ahead to elevate non-viral methods to the same status as AAVs. This includes attaining the most coveted prize of all: an FDA-approved treatment using non-viral delivery. AAVs are already FDA-approved, perhaps because they are currently ten to one hundred-times more efficient than non-viral delivery methods. “The big question with non-viral delivery is: is it going to be possible to get the efficiency needed to have therapeutic effects?” Murthy says. “It’s an open question whether or not we can improve the efficiency to make it clinically viable.” Nonetheless, researchers around the country, and companies like GenEdit, have already achieved great success in improving non-viral methods
Other remaining obstacles
Despite the progress in developing delivery methods for gene therapy, several obstacles remain for both ex vivo and in vivo delivery.
One prominent issue for ex vivo delivery is the price and difficulty of introducing DNA or Cas9 into cells. “The only clinical-grade device filed with the FDA to introduce any editing reagent into cells is an electroporator called MaxCyte. It is large, unwieldy, and difficult to work with,” Urnov says. A significant problem with electroporation is that it requires that cells from patients are exposed to open-air before they are electrically shocked to allow in DNA or Cas9. The consequences of open-air exposure are costly: “If cells are made to see open-air, in order to put them back into a subject, you have to utter the three most dreaded letters in all of gene and cell therapy: ‘GMP.’ Good manufacturing practice. And every time you say ‘GMP,’ you add three to four zeros to the price of anything,” Urnov says.
GMP is a set of standardized procedures to help control the quality of reagents that are used in multiple health products such as pharmaceuticals and medical devices. Although essential for the safety of patients, these practices can often be prohibitively expensive and time-consuming. A patient’s own tissue is not subject to GMP when inside a body, but as soon as it is removed from the body and exposed to a laboratory setting, the strict and pricey GMP rules apply.
Although in vivo delivery avoids exposing cells to open air, more work must be done to overcome concerns with AAVs and non-viral carriers. The AAV is only able to carry a small amount of DNA, making it hard to transport whole genes or more complicated treatments to a given cell. Non-viral delivery researchers, meanwhile, are struggling to decrease the size of non-viral carriers made of synthetic molecules. CRISPR-Gold, for example, has mainly been shown to repair DNA in the muscles and brains of mice, because both tissues can be easily targeted using local injections. Most non-viral CRISPR methods are so big that they are unable to be taken up by cells naturally when moving through the bloodstream. Now, labs—including Conboy’s and Murthy’s—are working on creating smaller non-viral delivery methods by attaching Cas9 protein with its guide RNA and donor DNA using small molecular linkers.
The promises of gene therapy
Gene therapy has made incredible progress from its deadly failures in the late 1990s and early 2000s thanks to continuing advances in electroporation, evolved AAVs, and gold nanoparticles. Since the beginning of 2020, at least nine conventional gene therapies have been approved worldwide. The number of traditional and CRISPR/Cas9-based clinical trials and approvals are only expected to increase in the coming years.
Gene therapy has completely changed the lives of those who have been cured by it. Victoria Gray, the sickle cell patient successfully treated using CRISPR/Cas9-based gene therapy in November 2019, has gone from occasionally being unable to move or even feed herself to thriving ten months later. She describes her cure as incredibly fortuitous, as she is now responsible for raising three of her children alone during the COVID-19 pandemic while her husband is deployed out-of-state by the National Guard. Taking care of her family would never have been possible without gene therapy.
Sierra Lear is a graduate student in bioengineering.
This article is part of the Fall 2020 issue.