On April 24, 1980, San Francisco resident Ken Horne was diagnosed with Kaposi’s sarcoma. He died the next year and would later be recognized as having the first case of Acquired Immunodeficiency Syndrome (AIDS) in the United States. In 1981, five previously healthy men in Los Angeles were diagnosed with a rare form of pneumonia called Pneumocystis pneumonia (PCP). Experts were puzzled—PCP only infects people with weak immune systems. What was it doing in these patients? A Centers for Disease Control and Prevention (CDC) report detailed these surprising findings and suggested that the men were likely all exposed to something that suppressed their immune systems. In the following days, the CDC was inundated with reports from doctors around the nation witnessing similar symptoms in their patients. Three years later, Human Immunodeficiency Virus (HIV) was identified as the cause of AIDS.
While many researchers and agencies like the CDC began crucial work immediately, many members of the American public reacted with fear and ostracized those with HIV. In the United States, an entire generation of gay men was shaped by the epidemic. In sub-Saharan Africa, female sex workers were and are disproportionately affected. More than eight in ten Kenyan female sex workers were HIV-positive in 1986.
The outlook in those early years was dire. By 1986 the CDC reported 493,000 people living with HIV. Death tolls from AIDS-related illnesses grew each year. Vaccine trials failed, and the few available treatments had harsh side effects and limited effectiveness. The shock and sadness of death became part of life for those in the most affected communities.
Hope came in 1995—at least in the United States. A new type of drug called a protease inhibitor was approved by the FDA for use by people living with HIV. Protease inhibitors ushered in a new era of treatment that greatly extended life expectancy. Since then, continuous improvement and development of HIV medications has led to better disease control with fewer side effects. Most importantly, HIV is no longer a death sentence. While still incurable, HIV is now a chronic illness that can be controlled by medication. A 20-year-old with HIV in California can now expect to live another 53 years. In the 1980s, life expectancies were closer to 12 years after initial HIV infection, and one to two years after AIDS diagnosis.
But this story is far from over. Worldwide, 37 million people live with HIV. Despite decades of intensive research, no vaccine or cure has been found. Only six in ten people with HIV access treatment. In many regions of the world, the social stigma of having HIV is devastating. Still, the community of HIV researchers, treatment professionals, advocates, and people living with HIV is cautiously optimistic that the ongoing advances in prevention and treatment will “end AIDS as a public health threat by 2030.”
Know thine enemy: understanding HIV infection
When HIV enters the body, it quickly begins to make copies of itself. Within a month, a single drop of blood can contain more than one million copies of HIV. As it replicates, it destroys white blood cells, which are part of the body’s immune system. This early stage disease is called acute HIV syndrome and feels like a bad case of the flu. Within weeks, the immune system gears up to fight back. As a result, the number of white blood cells increases. The body is battling the virus, but it is ill equipped to win the war.
In the absence of medication, the virus gradually increases in number over the years, slowly killing white blood cells. Eventually, the number of white blood cells becomes so low that the body is susceptible to infections and diseases that do not typically affect healthy people. At this point, HIV has progressed to AIDS, and even minor infections can be fatal.
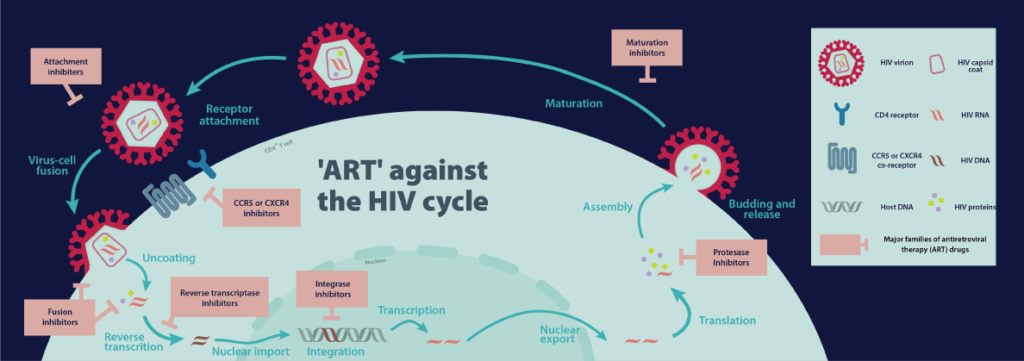
HIV mostly targets one type of white blood cell, the helper T cell. In a healthy immune system, helper T cells monitor the body, looking for potential invaders. The helper T cells are coated with molecular sensors which monitor the world outside the cell. Two of these sensors are called CD4 and CCR5. Scientists found that the outer coating of HIV—called the viral envelope—binds specifically to CD4. Once bound to CD4, the HIV envelope can also interact with CCR5. When bound to both CD4 and CCR5, the HIV envelope is able to fuse with the cell membrane and deposit its genetic information inside the cell.
The virus’s genetic code differs from a human cell’s genetic information: it carries RNA instead of DNA. As a result, it cannot directly use the host’s cellular machinery to make copies of itself. So, the virus uses a special viral protein called reverse transcriptase to convert its RNA into DNA. Now, the virus’s genetic material looks identical to human genetic material.
In a move that is both sneaky and very important for the success of HIV, the newly made viral DNA integrates itself into the host DNA.
The cell cannot distinguish between its own DNA and the viral DNA. It may ignore the viral DNA for a while, but sooner or later, the host cellular machinery uses it to unwittingly make copies of the virus. New copies of HIV bud off from the cell and embark on a mission to infect other helper T cells, and the process repeats. In their wake, the new copies of HIV leave a broken helper T cell that soon dies.
 This strategy makes HIV incredibly tough to cure. Eradicating the virus requires the destruction of every one of the millions of immune cells that contain HIV genetic material.
This strategy makes HIV incredibly tough to cure. Eradicating the virus requires the destruction of every one of the millions of immune cells that contain HIV genetic material.
Currently available medications, called antiretrovirals (ARVs), must be used in perpetuity to keep HIV in check. Some ARVs block HIV from binding to helper T cell sensors. Others block the activity of reverse transcriptase so that viral DNA cannot be made. Integrase inhibitors stop the viral DNA from integrating into and hiding in the host cell’s genome and protease inhibitors stop newly forming viruses from becoming mature.
Zidovudine (AZT), which blocks the activity of reverse transcriptase, was approved in 1987 as the first ARV for the treatment of HIV. However, HIV is a rapidly evolving virus, and as a result, 89 percent of people with late-stage HIV carried AZT-resistant virus within a year. In the early 1990s, doctors began prescribing combinations of ARVs. Evolving drug resistance became more difficult for the virus. Even if it evolved resistance to one of the drugs, another was still likely to stop it. The treatment of HIV with multiple ARVs is called antiretroviral therapy (ART), and it is now the standard of care for HIV.
While ART is necessary to control HIV long-term, the body does have a natural immune response to HIV. Cells have special guard proteins that monitor the interior of the cell, looking for suspicious material like viral proteins. One such guard is called the human major histocompatibility complex (MHC). If MHC finds a piece of virus, the complex grabs it, transports it to the cell surface, and waves it around to stimulate an immune response or initiate destruction of the cell.
A human protein called tetherin acts as another line of defense. Tetherin binds to newly developing HIV particles and, like a brick tied to the string on a balloon, keeps new virus from drifting away to infect other helper T cells.
Unfortunately, HIV can prevent MHC and tetherin from doing their jobs.
“If you’re the virus, you want to keep the tetherin and the HIV envelope apart from each other,” says Dr. Jim Hurley, Professor of Molecular and Cell Biology at UC Berkeley. “But if you’re the host cell and you want to defend yourself, you want to ensure that those things interact.”
Dr. Hurley’s lab studies how HIV orchestrates activity and movement within the cell to keep MHC and tetherin away from HIV. By studying the details of this orchestration, which involves physical interactions between HIV proteins and human proteins, Hurley hopes to find new opportunities for treatment. In addition, targeting the host-virus interaction may be a promising way to avoid drug resistance because human proteins evolve much more slowly than viral proteins.
“The key to [a new treatment] is to be able to target the interaction between the host and the pathogen without hurting the host and without giving the pathogen the opportunity to evolve resistance,” Hurley says.
Milestones in the fight against HIV
The outlook on HIV treatment dramatically changed in the mid-1990s. The first protease inhibitor was approved in 1995, and immediately, people on death’s doorstep improved dramatically. In 1996, the CDC announced that deaths from AIDS-related illnesses were decreasing in the United States. Persons with HIV realized they might live for years or decades longer than they had come to expect.
Seven years later, President George W. Bush announced the President’s Emergency Plan for AIDS Relief (PEPFAR) at his 2003 State of the Union address. PEPFAR greatly expanded access to life-saving HIV treatment in low- and middle-income countries. Before PEPFAR, about 50,000 people received ART in sub-Saharan Africa. Since then, PEPFAR has helped 13.3 million people access this life-saving treatment. As a result, millions of deaths have been prevented and HIV transmission from mother to child has been prevented in more than two million births. Now, PEPFAR is working toward controlling the epidemic in ten heavily burdened countries.
In 2007, an international team of researchers began a clinical trial to test the efficacy of an ARV called Truvada for preventing HIV. Truvada blocks viral reverse transcriptase, stopping the conversion of the viral RNA to DNA. Could Truvada perform that same function in a person exposed to HIV for the first time and stop that person from acquiring HIV?
They enrolled trial participants who were negative for HIV but at high risk for infection and, in 2010, the researchers announced that Truvada substantially decreases the risk of contracting HIV when taken daily. Truvada was approved for pre-exposure prophylaxis, or PrEP, by the FDA in 2012 and is recommended for individuals who are at high risk for HIV infection.
In 2011, prevention took another form when researchers empirically proved the efficacy of treatment as prevention. Prior to that study, there was no consensus as to when patients should begin ART: immediately after diagnosis or when helper T cell numbers dropped below a threshold. The study found that immediate treatment reduces the chance of transmission to a sexual partner. This is especially true if the HIV-positive partner is virally suppressed, meaning that ART has been so effective that the virus cannot be detected by standard measures in the blood.
In fact, a follow-up study found that a virally suppressed person with HIV has virtually no risk of passing HIV to a sexual partner. In 58,000 sex acts without a condom between a virally suppressed partner and an HIV-negative partner, the virus was not once passed to the HIV-negative partner. Treatment prevents the spread of HIV.
However, only 59 percent of people living with HIV receive treatment. Some are unaware of their HIV status, while others lack access or do not adhere to their prescribed treatment plans. UC Berkeley Associate Professor of Public Health Dr. Sandra McCoy studies approaches that can increase adherence to ART.
McCoy explained that people living in low- and middle-income countries face high social and economic barriers to HIV treatment. For some, shame and stigma prevent them from reaching out for care. Others travel to distant clinics where they will not be recognized. Pills are hidden in lotion bottles to avoid detection. And every day spent going to the clinic is a day of lost wages, which may compromise a person’s ability to meet their basic needs.
That’s why McCoy’s team researches “low-cost, scalable solutions that can improve adherence in these challenging settings.”
Recently, McCoy and colleagues conducted a study of people beginning ART to see if economic incentives improved clinic attendance and treatment adherence. Participants were given either food or cash incentives to attend the clinic each month. Both food and cash—but especially cash—effectively increased clinic attendance.
Providing food or cash is not cheap, but nothing about treating or preventing HIV has been cheap. Besides, McCoy says that “the real cost effectiveness is that for every person you get virally suppressed, you are stopping one person from spreading HIV.”
McCoy, like a huge swath of her colleagues in the HIV research and health care community, is working towards the 90-90-90 targets announced in 2014 by the Joint United Nations Programme on HIV/AIDS (UNAIDS). If successful, by 2020, 90 percent of all people living with HIV will know their HIV status; 90 percent of all people with a diagnosed infection will be on ART; and 90 percent of all people on ART will have viral suppression.
These goals were unthinkable just 10 years ago. Yet today, the 90-90-90 targets are considered attainable, if not by the original goal of 2020, then by 2025 or 2030. UNAIDS announced that in 2017, 75-79-81 was reached globally; 75 percent know their HIV status; 79 percent receive treatment; 81 percent are virally suppressed.
Ending the AIDS epidemic for good: developing a vaccine and the search for a cure
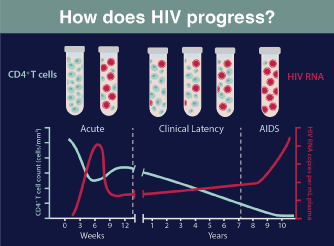 HIV RNA levels become detectable several days after primary infection and then increase exponentially until they reach a peak several weeks later. At this point, the adaptive immune response leads to partial control. Ultimately, untreated HIV infection causes the progressive destruction of CD4 + T cells in the blood and leads to a wide range of immunological abnormalities.
HIV RNA levels become detectable several days after primary infection and then increase exponentially until they reach a peak several weeks later. At this point, the adaptive immune response leads to partial control. Ultimately, untreated HIV infection causes the progressive destruction of CD4 + T cells in the blood and leads to a wide range of immunological abnormalities.
After HIV was identified as the cause of AIDS, researchers began working to develop a vaccine. In 1987, the first ones tested showed lackluster results and rapidly tempered optimism. In ensuing decades, dozens of vaccines have been developed, but failed to protect against HIV. Only one clinical trial has shown any efficacy at all, reducing infection by 31 percent in those who received the vaccine.
Vaccine development for HIV is challenging for the same reason that developing a single HIV treatment is difficult: HIV evolves rapidly. Most of the previously tested vaccines tried to teach the immune system to make guards called antibodies that recognize HIV markers and flag the virus for destruction. There are many varieties of quickly mutating HIV markers, and the guards fail to recognize most of them. So, in vaccines tested to date, the virus has been able to slip past the guards unnoticed. More guards that recognize a broader range of HIV markers are needed for an effective vaccine.
Now, in a new trial, researchers are trying a different approach. Instead of stimulating the body to make antibodies, they are delivering a special type of antibody, called broadly neutralizing antibodies, or bnAbs, directly to the immune system through an IV drip. BnAbs are antibodies that neutralize a broad variety of HIV strains. A small percentage of people with HIV produce them over time. They are superior guards, recognizing many versions of the mutating HIV markers. Researchers hope that these antibodies will protect people against a wider range of HIV.
An effective vaccine would provide a much-needed tool to contain the epidemic and prevent further infections. But even the best vaccine cannot reverse infections in the 36.9 million people living with HIV today. Even people who are virally suppressed are not cured, and ART must be taken daily to avoid viral rebound. The burden and expense of daily ART use are substantial over the course of a lifetime, especially in locations without easy access to medication. As a result, finding a true cure remains a top priority.
Currently, research for a cure revolves around two different strategies. The first strategy coaxes HIV out of hiding, then hits the virus hard with medication. The second approach uses newly-developed gene editing techniques like CRISPR to strengthen human resistance to HIV or to weaken or destroy the virus.
The former strategy, referred to as shock and kill, has two steps. First, a medication is delivered to wake up all of the human cells that have HIV lying latent within. When all of the cells with HIV are awake, medications are given to kill the virus. These drugs amplify the immune response to HIV. The hope is that the entire reservoir of latent cells can be woken up and destroyed at once. However, results from the first randomized controlled trial of this approach were disappointing. So far, researchers found no difference in the number of latent HIV cells between the experimental and control groups.
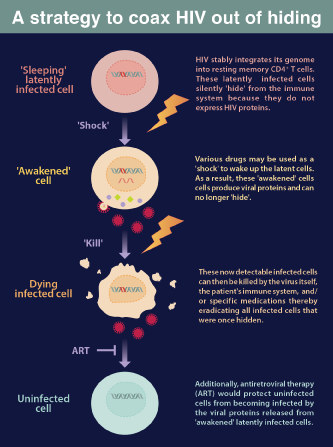 Still, work is ongoing to find new and better ways to shock and kill HIV. Dr. Qiang Zhou, Professor of Molecular & Cellular Biology at UC Berkeley, researches ways to coax HIV out of hiding. His lab has found that increasing the activity of some host proteins inside the sleeping cells can encourage the activation of latent HIV (see “Baiting the bug” Berkeley Science Review, Spring 2017).
Still, work is ongoing to find new and better ways to shock and kill HIV. Dr. Qiang Zhou, Professor of Molecular & Cellular Biology at UC Berkeley, researches ways to coax HIV out of hiding. His lab has found that increasing the activity of some host proteins inside the sleeping cells can encourage the activation of latent HIV (see “Baiting the bug” Berkeley Science Review, Spring 2017).
The second approach to cure HIV relies on a new gene editing technique called CRISPR. CRISPR has received national attention as a strategy to address many diseases, including HIV. CRISPR essentially acts as a pair of molecular scissors, inducing targeted mutations in DNA.
Researchers believe that CRISPR could be used to mutate host genes that HIV needs for its life cycle. For example, CCR5, one of the helper T cell sensors, could be changed so HIV can no longer recognize it.
Alternatively, CRISPR could be used to directly remove HIV genetic material from a host cell. Joe Hiatt, an MD/PhD student at UC San Francisco, is cautiously optimistic about using CRISPR to cure HIV.
“The power to use gene editing to realize an HIV cure is great,” Hiatt says, but “gene editing has a whole host of potential complications that need to be considered very seriously.”
He explained that because a person can now live with HIV for decades, any gene editing-based HIV cure must clear a very high safety and efficacy bar. Technical challenges remain as well, such as targeting the CRISPR machinery to the correct cell type, and ensuring that every single cell with DNA from HIV is reached. Even now, scientists still are not sure of all the locations in the body where infected cells lie latent. Finally, a CRISPR-based cure would likely be expensive, raising concerns about access.
Looking forward
More than 75 million people have become infected with HIV since the start of the pandemic. Over 35 million have died from AIDS-related illnesses. Enormous global efforts have resulted in huge improvements in the quality of life for people living with HIV. An HIV diagnosis is no longer a death sentence for people with access to treatment. Still, the fight must continue. Mercy Ngulube, a young woman who was born with HIV, said at the 2018 International Aids Conference, “[The] theme of complacency rings louder than any other challenge in this fight and it has to stop … We have the tools we need to fight this fight and do it well.” Recognizing the tremendous progress in the prevention and treatment of HIV is powerful because it provides motivation to continue pressing forward. And so, the research continues.
Becky Mackelprang is a postdoctoral fellow in plant and microbial biology.
This article is part of the Fall 2018 issue.