The Tinkertoy Construction Set was created in 1914 by Charles H. Pajeau, and over the past century, the iconic collection of wheels, sticks, and various other pieces have entertained generations of children. While the materials have changed over time from wood to plastic, and new pieces have been introduced, the Tinkertoy Construction Set remains at its core a series of sticks and wheels that can be assembled in an infinite number of ways, limited only by the imagination of its users.
Eighty-five years later, UC Berkeley Professor of Chemistry Omar Yaghi adapted the Tinkertoy assembly approach to molecular chemical compounds. In 1999, Yaghi published the first report of metal-organic frameworks, or MOFs, which use metals and organic linkers as their building blocks to form larger structures. Most importantly, these MOFs are highly porous and have a large surface area, which means they contain many locations to capture gas molecules or do new types of exciting chemistry. Yaghi and Jeff Long, another UC Berkeley chemistry professor, have continued to make fundamental discoveries to better understand and utilize MOF chemistry. Today, additional research progress is made by companies such as Mosaic Materials, led by UC Berkeley alumni Thomas McDonald, that are working to apply MOFs to a wide variety of real-world applications, including gas separation and storage, drug delivery, water collection, chemical catalysis, and more.
MOFs: chemical building blocks
 MOFs are based on two components: the metal-containing clusters and organic linkers. The metal clusters act as “wheels,” where holes represent the available binding sites for “sticks,” the organic linkers. In MOF-5, shown here, the metal cluster is an octahedron with six holes, one for each linker to bind to. The alternating SBUs and linkers create the extended three-dimensional MOF.
MOFs are based on two components: the metal-containing clusters and organic linkers. The metal clusters act as “wheels,” where holes represent the available binding sites for “sticks,” the organic linkers. In MOF-5, shown here, the metal cluster is an octahedron with six holes, one for each linker to bind to. The alternating SBUs and linkers create the extended three-dimensional MOF.
Like chemical Tinkertoys, MOFs are composed of two main components. The “wheels” are metal-containing clusters called secondary building units (SBUs), and the “sticks” are organic linkers that connect these clusters. To assemble a MOF from these pieces, scientists can simply mix and heat them together for a few hours or days. Over time, the parts assemble to form the repeating cages that make up the MOF structure. While each MOF requires a slightly different recipe, they are all essentially just different combinations of SBUs and linkers.
“Because of how they are synthesized, you can create MOFs that do just about anything that you can imagine for porous materials,” explains Jeff Martell, a postdoc in Long’s group. To create sites that trap or chemically convert gas molecules within a MOF, for example, researchers can tailor each SBU to be composed of two or three distinct metals that interact differently with the gas. Similarly, by varying the different organic linkers that join the SBUs, creating what are known as multivariate MOFs, scientists can create MOFs that are extremely stable, change color in response to different chemicals, selectively trap and release gas molecules, or have other interesting properties such as conductivity or flexibility. This customizability sets MOFs apart from other porous materials—which have traditionally been used for gas capture but allow for significantly less optimization—and has led to hundreds of thousands of distinct MOFs that can be applied in a wide variety of applications outside the lab.
New MOFs inspire creative uses
Since their first discovery in 1999, MOFs have been a prime candidate for gas capture, as their natural porosity and high surface area provides many locations for gas molecules to adsorb, or stick, to. Over the past 20 years, researchers have begun looking at other ways to take advantage of MOFs' customizability for other applications. The unique properties and tunability of MOFs have made them a hot target for researchers looking to bridge the gap between benchtop laboratory chemistry and real-world applications. “When we see a new sort of chemistry, we also then think, ‘Is there a use for it?’” explains Long. The relative ease with which scientists can build almost any custom MOF allows them to create robust materials to solve a wide variety of problems. In labs at UC Berkeley, scientists are pushing the boundaries of MOF chemistry to develop cutting-edge materials with new, creative functionalities.
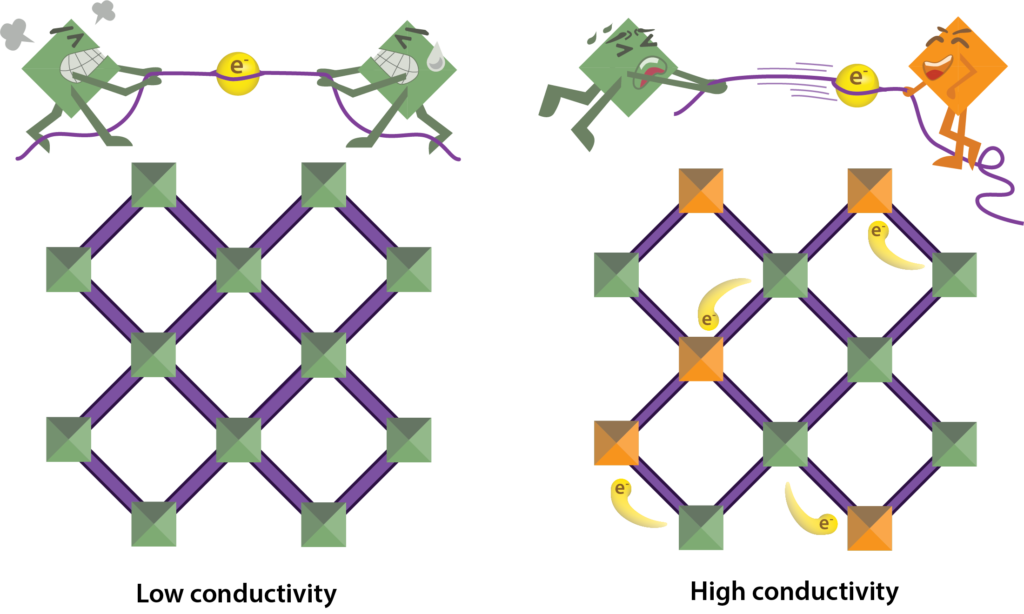 Electrons (spheres) travel more easily between metals with different electronic states (squares). When two metals have the same electronic state, the electron tends to stay put (left). When two metals have different electronic states, the electron will continue to move throughout the material (right).
Electrons (spheres) travel more easily between metals with different electronic states (squares). When two metals have the same electronic state, the electron tends to stay put (left). When two metals have different electronic states, the electron will continue to move throughout the material (right).
In Long’s research group, one of their ongoing projects is to make highly porous MOFs conduct electricity, which would create new specialized materials for thermoelectric devices, fuel cells, or batteries. However, at a fundamental scientific level, conductive MOFs seem counterintuitive. In principle, a good conductor allows charged ions or electrons to move freely through a material in a series of collisions—like billiard balls knocking against each other—passing electric charge as they move. These collisions are maximized when the material has a large cross-sectional area that allows more electrons to carry current. The principle directly clashes with the structure of MOFs, however, which are naturally very porous and consist of mostly empty space through which electrons cannot travel.
To address the problem of empty space and create MOFs that can conduct electricity, the Long group has developed new MOF structures with SBUs containing metals that exist in two or more different electronic states. Each state contains a different number of electrons, which makes it easier for incoming electrons to move between the different SBUs as the metals switch from one state to the other. This mixture of electronic states among the metals provides a pathway for charge transfer throughout the porous MOF without the need for a larger conducting surface area.
Long’s group is particularly interested in combining these conductive properties with their MOFs that perform gas capture, which could allow for the MOF material to act as a powerful sensor. Ideally, when a gas molecule binds to the MOF, it would trigger a change in electrical conductivity and either send a signal to scientists or prompt another chemical reaction in the material. With conductive MOFs, it is possible not only to trap gases like carbon dioxide, but also convert them to useful chemical products to make fuels, plastics, and more by selectively delivering electrons to the MOF pores. Scientists in the Long group hope that these new properties will drastically expand the ways we use MOFs in the future.
Yaghi’s group has taken a similar approach to customizing their MOFs by varying the components of the SBUs to create new structures called covalent organic frameworks, or COFs. COFs use the same building block approach as MOFs but replace the metal-containing SBUs with entirely organic components such as carbon, boron, nitrogen, and oxygen. Yaghi explains that “COFs have extended the precision of organic chemistry and organic chemistry reactions beyond 0D molecules and 1D polymers into 2D and 3D,” meaning that chemistry which before could only be done in one dimension has been expanded to two dimensional and three dimensional materials. Since COFs are entirely organic molecules, they can be synthesized and manipulated in ways that were previously impossible with MOFs. Furthermore, compared to MOFs, COFs can be more lightweight, stable, and have potentially higher surface areas and volumes, thus making them more efficient for gas uptake and other applications.
The Yaghi group is exploiting the unique properties of COFs to create flexible materials with better molecular recognition, separation, storage, or expansion capabilities. In the past, to obtain flexibility in MOFs, scientists would use pressure to deform the organic linkers within a framework. However, repetition of these movements places stress on the material, ultimately breaking the linkers. To address the rigidity issue, Yaghi has used COFs in a process he describes as “molecular weaving,” which involves mechanically interlacing organic molecules together as if they were threads. “The reason I can bend a cloth is because the threads move with respect to other threads without unzipping,” he explains. Just like in traditional knitting, these COF “threads” can move back and forth in all directions without placing any real stress on the molecules themselves. This weaving produces materials that are ten times more flexible than unwoven COFs. While the research is still in its early stages, molecular weaving opens the field to a whole variety of exciting new applications, such as flexible solar cells or electronics.
MOFs in action: carbon dioxide and water capture
The MOFs used for gas capture have come a long way since Yaghi’s first discovery 20 years ago. Researchers around the world have designed many new MOFs and are using them to capture gas molecules from industrial power plants or automobiles.
In 2015, while investigating MOFs for the capture of carbon dioxide, researchers in Long’s group discovered a specific MOF that, under a specific temperature and pressure, was very effective at selectively capturing carbon dioxide. Furthermore, it exhibited cooperative capture—meaning that the capture of a single carbon dioxide molecule triggers a chain reaction in the material that makes the capture of additional carbon dioxide molecules more favorable. This process is fully reversible with only a small change in conditions, which allows scientists to capture and release carbon dioxide in the MOF at will. More recently, the Long group has shown that MOFs can perform similar cooperative capture mechanisms for carbon monoxide and are working to extend the system to other key industrial gas molecules.
As mentioned above, MOFs need to be able to perform multiple functions at the same time to be useful for real-world applications. The goal for Yaghi’s team is to have a single MOF that can capture carbon dioxide, concentrate it, and then transform it into another chemical like carbon monoxide, which can later be used to make valuable chemical products. This has direct applications in industry, where MOFs can be used to first separate carbon dioxide from an industrial gas stream. Then, catalysts incorporated into the MOF’s organic linkers can be used to convert it to other chemical products, often by absorbing light and using that energy for conversion.
Different MOFs and COFs can also be customized to maximize their carbon dioxide capture in different environments. Materials that can capture carbon dioxide at low concentrations, for example, are needed in sealed spaces like spacecraft and submarines, where even low levels of the gas can adversely affect the occupants. For gas storage, MOFs can be used in an empty gas cylinder, where its highly porous nature allows it to store nine times the amount of carbon dioxide that would normally fit in the cylinder. Alternatively, for natural gas separations, selectivity is the key parameter. By varying the pore size of the MOFs or COFs, they can be designed to capture only carbon dioxide while allowing other gases such as methane and water vapor to pass through. This tunability plays an integral role as scientists explore the myriad of possible MOF applications.
While carbon dioxide and industrial gases are heavily studied, another key area of MOF research at Berkeley is capturing water from the atmosphere. Yaghi’s team recently built a MOF-based water harvesting device and optimized it for use in a desert atmosphere. The device, which is roughly the size of a microwave, is enclosed in an acrylic plastic case that allows for condensation, sunlight reflection, and heat transfer. During the night, the case is opened, allowing the MOF to be saturated with moisture from the desert air. During the day, the case is closed and sunlight powers the release of hot, humid air from the MOF to a condenser, which releases the heat to the surroundings and collects liquid water. Yaghi’s students have successfully conducted field tests in an Arizona desert with this device, producing up to 175 grams of water per kilogram of MOF during each day-night cycle, powered only by natural cooling and ambient sunlight. Compared to other water harvesting devices, the MOF device was more than five times as effective for producing water. As with many of the MOFs they’ve designed in the past, Yaghi and his team intend to commercialize the technology.
While all these MOF and COF materials look very promising at a fundamental level, there is still a lot of work necessary to demonstrate their use in actual commercial processes. As academic research laboratories, the Long and Yaghi groups don’t have the expertise needed to scale up production or push these materials to industry standards. “MOFs are these microcrystalline powders, and you need to turn them into a structured form, where the material can be easily accessed by gas but is also mechanically robust,” explains Long. These are problems that academic research groups simply can’t answer with so much of their time focused on developing the basic science behind MOF synthetic chemistry and fundamental materials characterization. For MOFs to be successful at the next level, it is critical to get a good feedback loop between the engineers designing an application and the scientists who understand the MOF chemistry and properties.
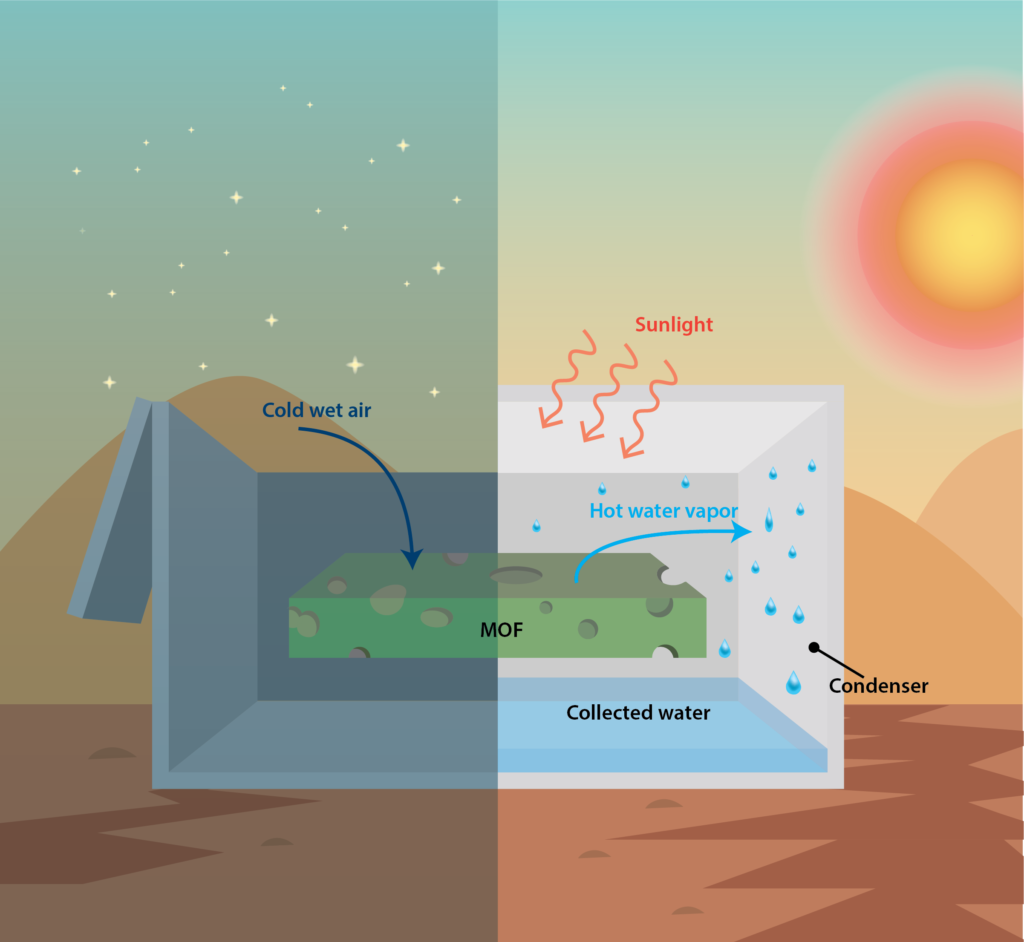 Scientists have developed MOF-based water harvesting devices to work in a desert atmosphere. In the evening, cold water vapor is taken up and stored in the MOF material. When the device heats up in the daytime by sunlight, the warmed vapor condenses and allows for water collection.
Scientists have developed MOF-based water harvesting devices to work in a desert atmosphere. In the evening, cold water vapor is taken up and stored in the MOF material. When the device heats up in the daytime by sunlight, the warmed vapor condenses and allows for water collection.
Taking MOFs mainstream
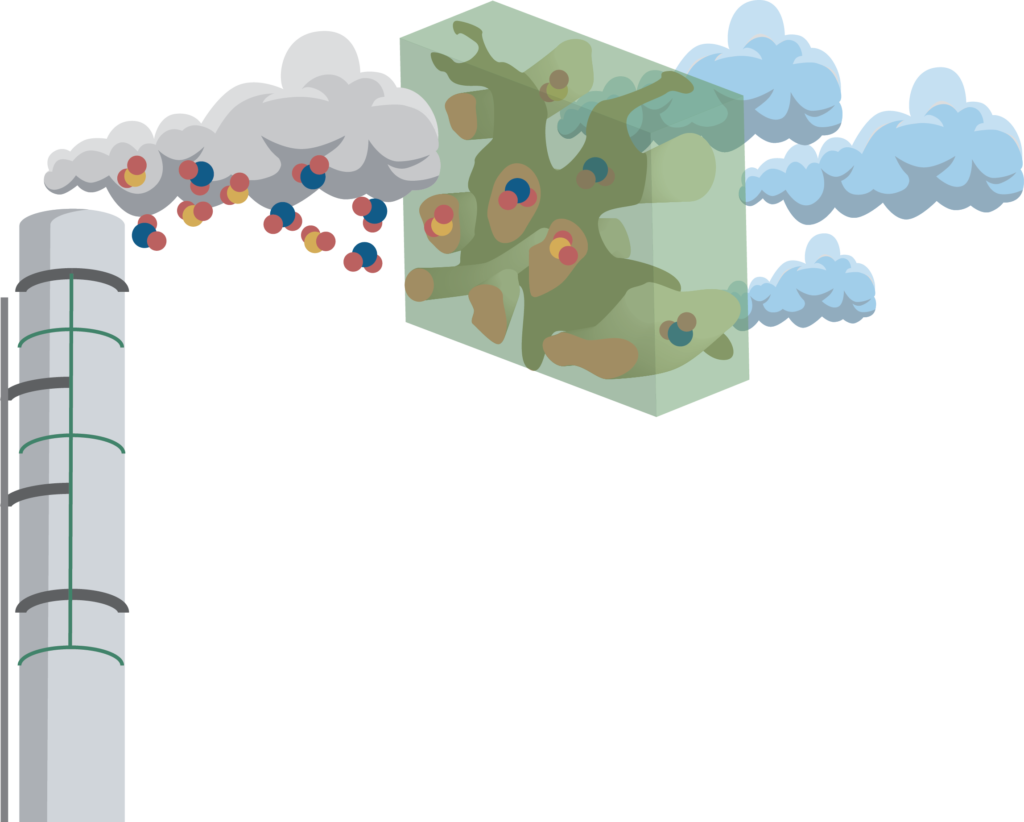 The combustion exhaust gas produced at power plants contains a lot of chemical contaminants, such as sulfur dioxide (SO2)and nitrogen dioxide (NO2 ). Mosaic Materials is developing commercial MOF products to selectively capture carbon dioxide from these contaminated gas streams.
The combustion exhaust gas produced at power plants contains a lot of chemical contaminants, such as sulfur dioxide (SO2)and nitrogen dioxide (NO2 ). Mosaic Materials is developing commercial MOF products to selectively capture carbon dioxide from these contaminated gas streams.
In Berkeley, you don’t have to look far to see the feedback loop between industry engineers and academic scientists in action, and optimizing MOFs for industrial carbon dioxide capture is a perfect example. A common obstacle facing emerging technologies is that the laboratory setting in which materials are tested is significantly less complex than the industrial environments they will be used in. For MOF gas capture, this often means that experiments focus on the ability to capture carbon dioxide without considering the huge impact of other air contaminants. Because of their desire to move MOF products into mainstream application, the Long group is careful to test their new MOFs with many contaminants like nitrogen, water, and other gases.
In 2014, Long and former graduate student Steven Kaye launched Mosaic Materials, a Berkeley startup company using MOFs for gas and chemical separations. Thomas McDonald, another former graduate student of Long’s, took over the company’s operations in 2015 after Kaye left. Mosaic Materials was admitted into the first cohort at Cyclotron Road, a two-year fellowship program at the Lawrence Berkeley National Laboratory empowering energy innovators to take their ideas from concept to commercial product.
The research focus at Mosaic Materials is to scale up the carbon dioxide and chemical separation techniques developed in the Long lab at low cost without losing the capacity and stability that made these MOFs stand out at the laboratory scale. “Finding materials that last just long enough to be commercially viable at the scales that are necessary given their manufacturing cost is our greatest challenge,” says McDonald. “It’s definitely a solvable problem, but it will take time, resources, and failure.” As a company that grew out of academic researchers, Mosaic Materials takes a materials-first approach, ensuring that their product is of the highest quality and provides substantial benefits to the end user. In McDonald’s words, “We know exactly the types of properties these MOFs should have and we hold ourselves to those high expectations.”
Mosaic Materials is not alone in this venture. In 2012, when the Berkeley Science Review last published an article on MOFs (see “MOFiosos” Berkeley Science Review, Fall 2012), MOFs had yet to be applied in the real world. Now, Yaghi estimates that there are at least two dozen small companies, many of which also spun out of academic research groups, working on commercializing MOFs for a variety of applications. NuMat Technologies, founded on research done at Northwestern University, was one of the first—launching their MOFs for hazardous gas storage in the electronics industry in 2012. In the UK, MOF Technologies created several MOF products to prevent fruit ripening and collect heat from large-scale computing centers. In the healthcare industry, MOFgen has developed coatings that can store and release antimicrobial agents. Back at UC Berkeley, BASF, a German chemical company that was one of the early adopters of MOF technology, has worked with Yaghi to develop a MOF-based natural gas fuel system for vehicles that is currently in the testing stage.
Despite Mosaic Materials being much younger than these other companies, McDonald hopes to have a field pilot of large-scale MOFs for carbon dioxide separation installed within a year. In five to ten years he hopes to have hundreds of tons of MOFs being made for energy-related applications, solving some of the world’s toughest problems, including carbon dioxide capture, chemical production, and chemical catalysis.
MOFs: looking ahead
 Mosaic Materials is working to create robust MOF products in large-scale quantities with various shapes and sizes that can be used to fight air pollution.
Mosaic Materials is working to create robust MOF products in large-scale quantities with various shapes and sizes that can be used to fight air pollution.
For the last 20 years, the number of MOFs has been growing exponentially, with over 80,000 MOF compositions now known and hundreds of groups around the world studying them. Yaghi offers an explanation for their popularity: “Relatively speaking, this building block approach to chemistry works. You can imagine a structure, then go to lab and make it.” To share the chemistry of MOFs and introduce more young scholars to research, Yaghi is a proud sponsor of the Laboratory Research Experience program that brings students in their junior year of college to Berkeley to immerse them in lectures from distinguished speakers and hands-on laboratory experience. He has also helped start MOF research labs in Japan, Korea, Vietnam, Mexico, Malaysia, and Saudi Arabia, with plans for more in Kuwait, Jordan, Kazakhstan, Peru, and Argentina. Many of the existing labs now operate independently and provide a place for emerging scholars to learn how to become MOF scientists.
These new scientists will be critical in helping tackle the biggest challenges still facing MOFs. As more MOFs become commercially viable, these scientists must be able to explain the potential of MOFs to people in industry. “There are unique applications where MOFs can have a big advantage over other porous materials, but people have to understand how they work and what their characteristics are,” explains Long. In conjunction, there simply needs to be more work done on the commercialization front. McDonald suggests that the next major MOF developments will be at the “practical, applied level much more so than the sky-high ambitions.” With challenges such as climate change growing more pressing every day, all of these approaches at Berkeley and around the world will be critical to help MOFs make important contributions in energy, health, manufacturing, and more as they become part of our everyday lives.
Christopher Jackson is a graduate student in chemistry.
This article is part of the Fall 2018 issue.