Jeanne Calment died at the astounding age of 122 years, 3 months, and 6 days—the oldest recorded living person. Jeanne was reputed to still be smoking, drinking, and indulging in her native rich French cuisine well into her triple digits. Given the rarity of such longevity, why did Jeanne live as long as she did? Perhaps it was the extraordinarily good luck in the genetic cards she was dealt at birth, or something else altogether. The cause of her death in 1997 is unknown. With recent advancements in aging research, scientists are on the cusp of making a lifespan as long as Jeanne’s the norm rather than the exception. Researchers are beginning to uncover important causes of senescence and longevity, as well as possible targets for intervention. In the dawning era of anti-aging therapeutics and genome engineering, it’s likely that life-extending treatments could be hitting the shelves soon. However, these advances do not come without significant controversy.
“Aging encompasses everything,” with implications for society, medicine, and even economics, says Dr. Andrew Dillin, UC Berkeley professor and co-director for Paul F. Glenn Center for Aging Research. Treatment for degenerative aging diseases accounts for a staggering 80 percent of the multi-trillion-dollar US healthcare industry. Predictions for quality of life are grim, as the drug treatments available are demonstrably ineffective, and human life expectancy is increasing globally. As we plod arthritically through an onslaught of age-related disease—cancer, Alzheimer’s, cardiovascular disease—it seems only a matter of time and odds which will kill us first. These ticking time bombs are intimately intertwined with our livelihoods, and with those of our grandparents and children. Finding cures for seemingly unavoidable diseases has been the focus of medicine for centuries, and researchers are closer now than they’ve ever been to making aging obsolete.
How and why we age
While there is no universally accepted definition of aging, many describe it as a gradual accumulation of molecular and cellular damage. Aging is like a microcosm of entropy, the progressive, inevitable decline of order into disorder. The clocklike nature of the aging process is coordinated enough to be predictable. As we age, the wrinkles deepen, the waistline expands, and the risk of dementia and type 2 diabetes synchronizes with the gradual accrual of years.
A number of critical factors behind aging have been identified over the past several decades. Their relative importance is poorly understood, however, and likely varies between individuals due to family history and living conditions. A large part of the story lies at the microscopic level in cell division. As cells replicate and divide, they must copy all of their DNA and split it between parent and daughter cells. In this way, one cell becomes two cells, two become four, and so on until they comprise the trillions of cells that make up our bodies. Each of these cells is also replicating and dividing, upwards of tens of thousands of times for certain cell types. The number of things that could go awry is statistically worrisome, but there are only a limited number of divisions a cell is
capable of before its death. This restriction is referred to as the Hayflick Limit, named after Dr. Leonard Hayflick of the UCSF School of Medicine.
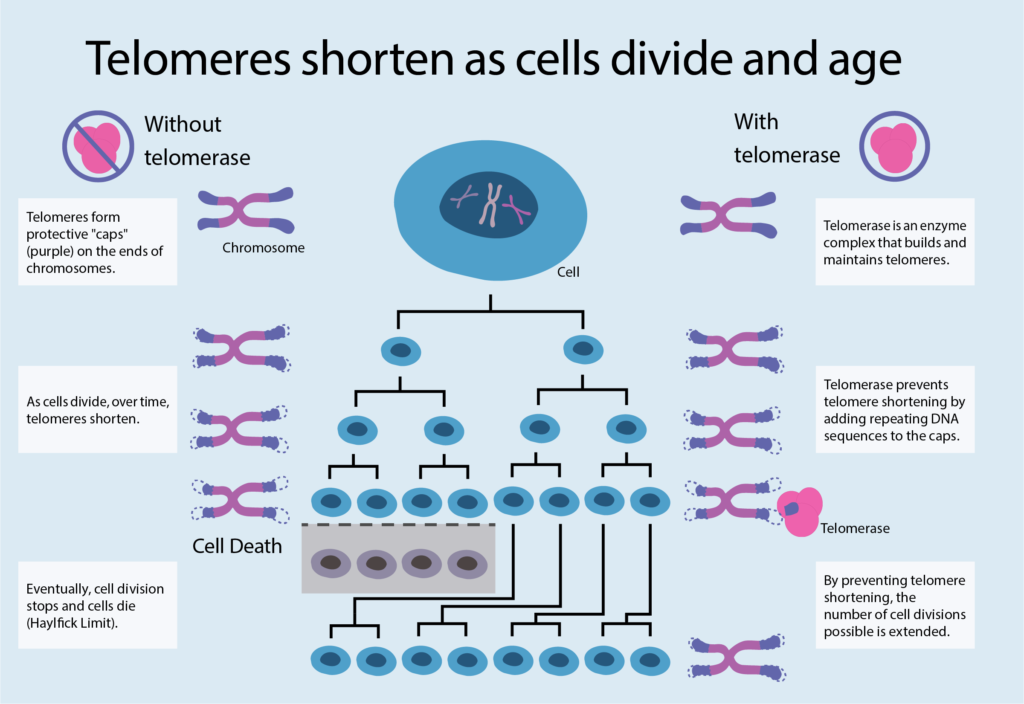
At the heart of this finite replication capacity is the telomere. The telomere is a region of DNA at the end of the chromosome that protects the chromosome from damage as cells replicate and divide—like a cap on your shoelace that prevents it from fraying. Telomeres gradually shorten over the organism’s lifetime as progressive cell divisions take place up to the critical point at which division is no longer possible and aging and cell death are imminent. Telomere shortening has been implicated in many of the age-related diseases we eventually succumb to. For some diseases, short telomeres can even be used as a diagnostic tool. But there’s more to predicting your lifespan than the length of your telomeres alone. The rate of shortening is also important, as well as your body’s ability to maintain cellular functions and prevent damages from accumulating.
Telomere shortening can be counteracted by telomerase, an enzyme complex that prevents your chromosomes from unraveling by adding repeating DNA sequences to the telomeric caps, thus extending the number of cell divisions possible. Professor Kathleen Collins of UC Berkeley chose to study telomerase for her postdoctoral research. Practically no one else was working on it at the time. “It’s one enzyme,” Collins recalls thinking. “How complicated could it be?” Almost 26 years of research later, it turns out that telomerase is both incredibly complex and important. “In fact, it’s more complicated than an average enzyme,” says Collins. “It has many chemical and structural intricacies behind its otherwise simple function.”
Research on telomerase initially began in the pond scum Tetrahymena. These unassuming, single-celled protozoans can avoid aging and death altogether, making them, in effect, immortal. Tetrahymena make a lot of telomerase, which prevents their telomeres from shortening, allowing cells to divide indefinitely. Increasing telomerase expression in other organisms has also been shown to increase cell lifespan almost infinitely.
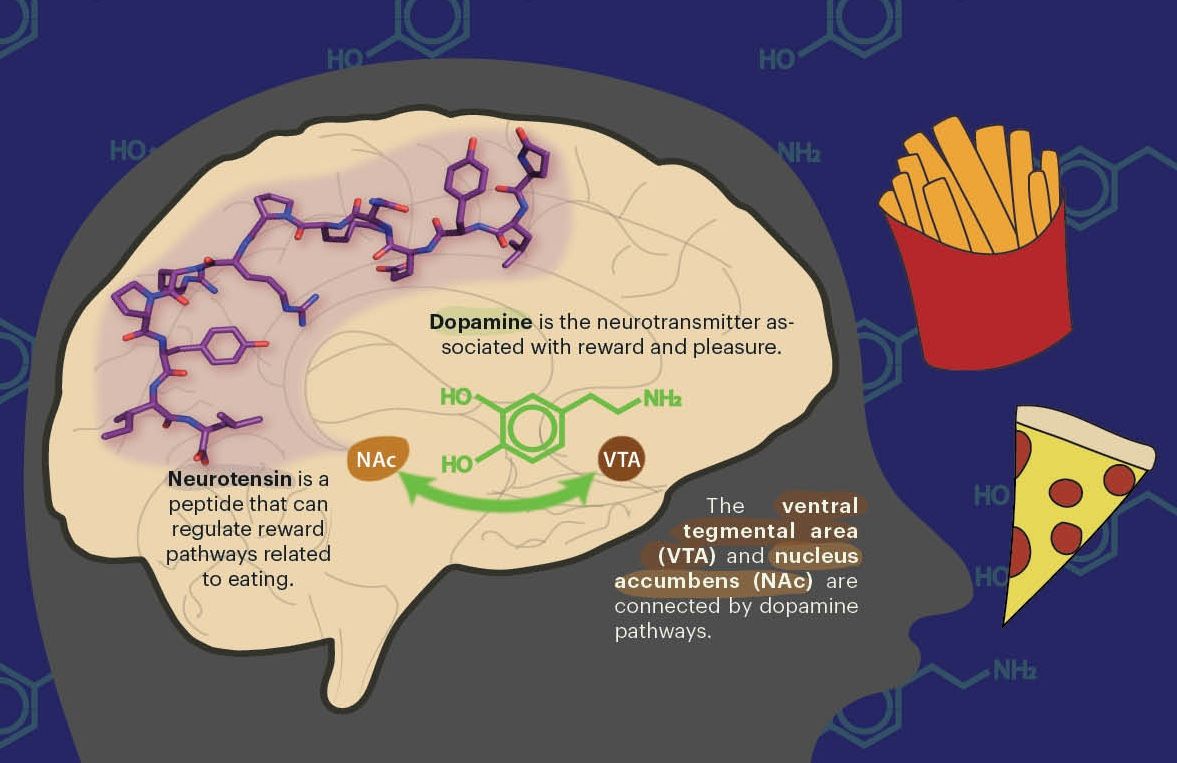
Unfortunately, reversing human aging is not as simple as increasing telomerase activity. The enzyme is mainly active in early human embryos, and as the embryo matures, there is a large developmental switch that turns telomerase off almost entirely. Telomerase is scarce in adult cells, barely active for the remainder of the organism’s life and often not enough to compensate for successive telomere shortening. The reason behind this apparent cellular death wish is cancer prevention. Almost all cancerous cells have hyperactive telomerase that enables their rapid and unlimited growth. Altering telomerase’s careful balance in pre-cancerous cells could induce the disease.
Telomere shortening is far from the only cause of aging, of course. There are a number of other contributors, such as DNA mutation, stress, and accumulated tissue damage, whose additive effects can also be fatal. Scientists have only just begun to shed light on some of these complex processes, and any medical treatments are still a long way off from clinical trials.
There is also the question of why we age in the first place. Biological immortality is not just found in Tetrahymena pond scum—it’s also present in some of our cells. Germline cells (eggs and sperm) and stem cells are immortal. The somatic cells that comprise the rest of our bodies, on the other hand, are not. Each life begins as a single cell that, like a stem cell, can proliferate without death. Somatic cells branch from this immortal lineage, then lose the unlimited capacity for cell replication and division, likely to suppress cancer.
Lisa Triedel, a PhD student in the Department of Integrative Biology, explains that there are many hypotheses about the evolutionary origins of aging. A unifying theme is that the effects of aging are often felt most after the reproductive years have passed. Not only is the individual older, the evolutionary consequences of accumulated damage are much lower after genetic material has already been passed on. There’s also a discrepancy between how much costly maintenance one’s body will invest into repairing heritable germline cells versus the non-heritable somatic cells. The invisible thread of immortal germline cells that connects us across generations is manifested in our disposable bodies, all as a means of reproduction.
Could (and should) we intervene in human aging?
The rapidly emerging field of anti-aging therapeutics has been spurred on by both the human desire for longevity and recent advances in genomic engineering. The two main avenues for therapy are either to engineer an embryo to improve its initial genetic quality, or to intervene later in life at the onset of aging symptoms. For a variety of medical and ethical concerns, the latter is likely the more feasible option. Researchers can identify the various causes of aging, and while they can’t stop them outright without potentially unintended and profound consequences, they might be able to delay them or reduce their severity.
AgeX Therapeutics, a biotechnology company in Alameda, California, is attempting to redefine the way we age. AgeX, a subsidiary of BioTime Inc., was created in 2017 to develop and commercialize life-prolonging therapies. “There are many presuppositions of how the body breaks down—that it is irreversible,” says AgeX CEO Dr. Mike West. “We believe that the biology of aging will surprise people.” As former CEO of Geron Corporation (famous for cloning the first animal, Dolly the sheep), West gathered acclaim in the regenerative biology field when he discovered a gene capable of immortalizing somatic cells through a process called telomerase therapy.
The active component of the telomerase enzyme complex is controlled by a single gene, called hTERT, acting like a master switch. The hTERT gene is active in stem cells but often not in somatic cells. hTERT was successfully transferred from an immortal cell line into a somatic cell line, for which Dr. Hayflick donated a piece of his own skin. This transfer indefinitely increased the cell’s replicative lifespan without it becoming cancerous. West was at dinner with Hayflick when he broke the news that this single gene might be the key to immortality. Hayflick was said to be silent for some time, saying at last, “It can’t be that simple.”
While it may seem far-fetched, the goal of AgeX is to commercialize longevity. Regenerative medicine’s aim is not to interfere with how the damage happens, West explains, but to deal with its consequences. This immortalization process in particular could have far-reaching applications for a number of age-related diseases, including macular degeneration, cardiovascular disease, and arthritis. In a procedure as simple as taking your car in for periodic repairs, this therapy could work to reduce damage accumulation and restore populations of young, healthy cells. It’s imperative to have control over this technology before implementing it, AgeX acknowledges, particularly when we’re messing around with a process as complex as aging.
“Don’t get the world thinking that a telomerase therapy will cure aging,” cautions Collins, who is skeptical about the efficacy of this treatment. Some critics have lambasted this therapy as misleading marketing at best, aimed at selling costly and ineffective products to an upper class thirsting for perpetual youthfulness. At worst, scientists warn that the cancer risks could be overwhelming.
 West says cancer cells are “like a runaway car: brakes broken, gas pedal stuck to the floor, and an infinite fuel supply.” Treating cells with telomerase could rejuvenate aging cells, or backfire and turn pre-cancerous cells cancerous. When applied to targeted non-cancerous cells in aging tissues, though, such telomerase activation therapy is very promising. “Clinical trials for this could be well on their way in the next five years,” says Collins.
West says cancer cells are “like a runaway car: brakes broken, gas pedal stuck to the floor, and an infinite fuel supply.” Treating cells with telomerase could rejuvenate aging cells, or backfire and turn pre-cancerous cells cancerous. When applied to targeted non-cancerous cells in aging tissues, though, such telomerase activation therapy is very promising. “Clinical trials for this could be well on their way in the next five years,” says Collins.
On the other hand, turning telomerase down or off altogether in cancerous cells could force the runaway car to a screeching halt. “Telomerase inhibition could cure cancer,” says Collins. One of the most promising directions Collins’ research is heading in is the search for such a molecular inhibitor that could be taken orally as a standard drug. “We’re getting really close,” says Collins, “but it’s still a ways off.”
“Telomerase therapy is not for everyone,” warns Collins. The role of telomerase can vary across a wide assortment of age related diseases, and the same disease could be caused by different factors in different people. For those suffering from chronic declines in function associated with shortened telomeres, however, this therapy could extend their healthy lifespans by an order of decades. “But we’re not going to create immortal people by increasing telomere length alone,” says Collins. “They’ll die of something else, like getting run over by a truck.”
There are also noninvasive procedures for extending lifespan, which can be as simple as managing your stress levels. A 2009 study from Kaiser Permanente and UCSF found that other factors such as socioeconomic status and lifestyle choices, like smoking and alcohol consumption, also serve an important predictive role in how fast aging occurs. Stress—chronic, acute, psychological, physiological, or otherwise—has been repeatedly demonstrated to cause telomere shortening. Many studies have found that people with higher perceived stress levels have notably shorter telomeres and an increased risk of contracting age related diseases. Luckily, there is growing evidence that meditation and positive attitude can prevent telomere shortening in the long run.
So, while it’s increasingly feasible that we could delay or prevent aging altogether, should we?
“What a f—ing stupid question,” says Dr. Aubrey de Grey, AgeX’s Vice President. While so new to the company that his name plate is a folded up piece of paper taped on his office door, he is by no means new to the regenerative biology field. This vocal and often controversial proponent of human immortalization has decades of research experience on the subject. Twirling the ends of his extensive beard, this Cambridge-educated researcher discusses how the science is ahead of the funding. “There’s a fifty-fifty chance of ending aging in 20 years,” de Grey explains, attributing the public’s hesitation in investing in this technology to a deeply rooted psychological barrier. He describes a mutually reinforced belief that life would have no meaning without death. “Bollocks about death adding to life,” says de Grey, taking a beer from his briefcase and pouring it nonchalantly into a disposable coffee cup.
Other scientists and healthcare professionals are skeptical about the targets of such anti-aging therapies because they’re intimately linked with other cellular functions, often in ways we don’t yet understand. There are some genes that could be mutated to make animals live longer, but the unintended consequences could very well outweigh the benefits. While it may be possible to extend your lifespan or treat a particular age related disease, chances are that your overall quality of life will be poor.
The variation between individuals in the aging process could also severely complicate the effectiveness of commercial treatments. “We’re all going to age differently,” says Dillin. “[Aging prevention] is going to be individually personalized because aging is a massively complex issue.” Take a car for example: it indisputably ages, but the rate across models can vary widely. “Why does a Ford Focus have a lifespan of 10 years,” asks Dillin, “whereas a Mercedes can last for 20 or 30 years?” Maybe aging and its affiliated afflictions are more about how well the parts are assembled at the beginning, rather than the process itself. Maybe aging has more to do with the maintenance of the parts over time, or maybe it’s too hopelessly complex to know. Nevertheless, we’re only just beginning to understand some of the some critical individual variations in aging.
The risk of unintended consequences is also cause for hesitation—a caveat that was exemplified by Dillin’s most surprising research finding. Dillin’s aging research focuses on mitochondria, an energy-producing component of our cells that is critical in metabolism. The Dillin lab showed that a single, specific mutation in the mitochondria increased the lifespan in both C. elegans worms and mice. But what are the other effects on the animals? “They’re horrible,” says Dillin, for although the worms lived twice as long, their quality of life was probably half as good. There were many tradeoffs with a longer lifespan, including severe developmental and reproductive delays. With these targeted life-extending mutations, Dillin explains, “we can make the car look really good, but it’s like forgetting to put the wheels on. The engine and radio might work really well, but it’s not going anywhere.”
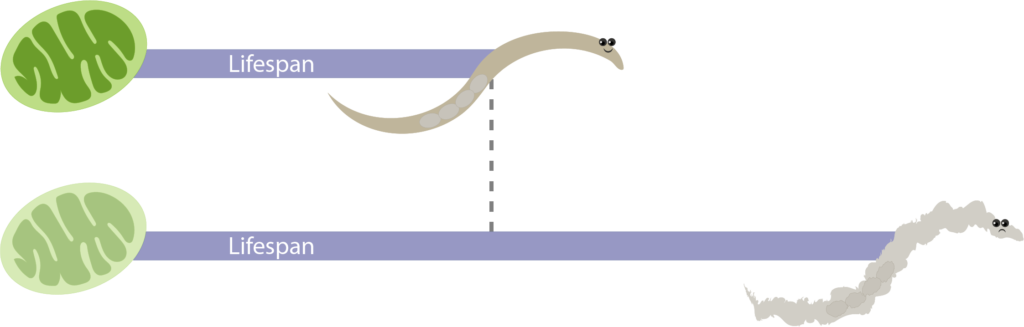 Research from Andy Dillin's lab here at UC Berkeley showed that a specific single mutation in the mitochondria, an energy-producing component of cells, doubled the lifespan of the worm model organism, C. elegans. However, even though these animals lived longer, the animals looked unhealthy and presented severe developmental and reproductive delays. Credit: Kurtresha Worden.
Research from Andy Dillin's lab here at UC Berkeley showed that a specific single mutation in the mitochondria, an energy-producing component of cells, doubled the lifespan of the worm model organism, C. elegans. However, even though these animals lived longer, the animals looked unhealthy and presented severe developmental and reproductive delays. Credit: Kurtresha Worden.
Transferring successful results from the lab to the real world has also proven elusive, as the world is far less hospitable than a petri dish. For example, while another targeted mutation made the worms live longer in the amiable lab environment, they actually died more quickly when placed in their natural soil habitat. These contradictory results could be due to unknown interactions between genes and their environment, or any number of things. When scaled up to the human level of complexity, and compounded with individual variation in aging, unintended consequences could significantly complicate and delay human trials. Aubrey de Grey concedes that treatments would have to be individually personalized for maximal effect.
From accidental health consequences to the still unknown societal implications, many scientists are fearful of meddling in an area where we have no rules. Misinformed or misguided application of aging research to humans could very well be catastrophic. It might be better to have therapeutics that target specific age-related diseases rather than aging itself. Anti-aging therapies might also further promote social inequality by being prohibitively expensive. Unless this technology is employed democratically, reserving it for the top 0.01 percent would severely widen the socioeconomic gap. But these anti-aging therapies could go the way of antibiotics and other breakthrough treatments: at first costly and reserved for the wealthy, these now widely available medications were made affordable by government subsidization. The resulting swell of longer lived populations would likely exacerbate current levels of environmental degradation.
AgeX’s de Grey is not concerned by the overpopulation issues related to immortality, as we’re dealing with exponentially growing human populations now and can very well continue to do so in the future. It’s difficult to conjure an entirely non-dystopian future for such a society, though. With potential triple-digit average lifespans, we would be stretching our already limited resources to feed the voracious appetites of a growing consumer hoard. Technological advancements alone would not be sufficient to prevent further environmental catastrophe, from increased pollution, climate change, disease outbreaks, famine, and mass extinctions.
There would be many things to reconsider as a society as well, such as how living for centuries might change the way we form relationships and raise our children, and how culture might progress if there were no generational turnover. The timespans of employment and prison sentences would have to be drastically restructured. Human behavior and interaction, free from the fear of impending illness and death, could be unrecognizable to us today.
Tampering with our genes in this way could blur the line between what is natural and unnatural in the future. Whether we engineer designer babies or intervene genetically later in life, the primeval appeal of extending lifespan makes it unlikely that there will be an outright ban of such technology. Moving forward, necessary precautions should be taken.
It’s also important to take longevity into perspective. Human lifespan has already doubled in the last century—the biggest leap we’ve made since the evolution of our species some two million years ago. We’re already taking and will continue to take drugs that improve our quality of life, from Ibuprofen to the newest cancer drug. Which prompts the question from Dillin, “Why do we need to live longer?”
Briana Boaz is a graduate student in integrative biology.
Design credit: Kurtresha Worden
This article is part of the Spring 2018 issue.