Did life evolve somewhere else? Of course,” says Richard Mathies, chemistry professor at UC Berkeley. Mathies is developing the technology to explore extraterrestrial life in our solar system and beyond. “What does it look like? I’m not sure.”
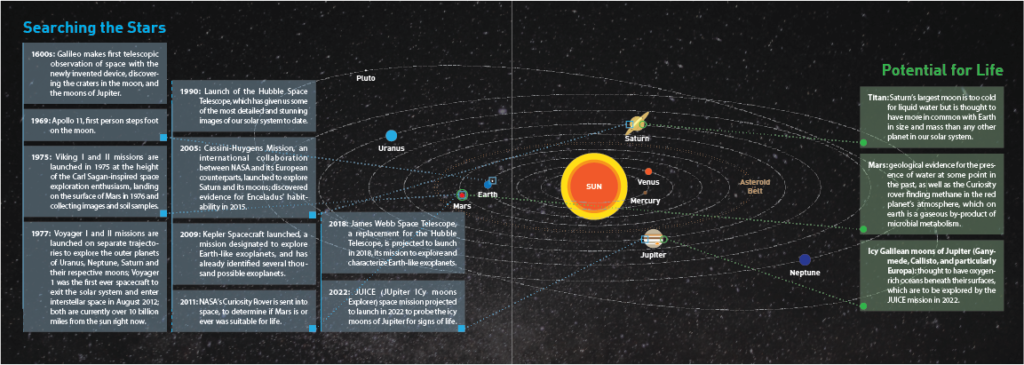
Recent advances in space exploration technology have led to an explosion of newly discovered exoplanets in our corner of the galaxy. The projected number of planets in the universe is an unimaginable 50 sextillion (50,000,000,000,000,000,000,000). These discoveries have expanded a field known as astrobiology, which seeks to answer such grandiose questions as the origin, distribution, and future of life in our galaxy and beyond.
Some of these 50 sextillion planets are likely to fit the Goldilocks requirements for habitability: that is, planets that have just the right temperature, pressure, and liquid water capable of supporting life as we know it. Even if the probability of life on any given planet is infinitesimally small, the large number of potential Goldilocks planets all but guarantees the existence of extraterrestrial life. Even in our immediate galactic neighborhood, around 10 percent of stars are orbited by Earth-sized planets within a habitable zone—that is, an orbiting distance from its star where water can remain liquid.
Life on Earth, in all of its many forms and degrees of complexity—from single bacterial cells to humans—follows the same pattern. Cells require carbon. Water is necessary for the biochemical reactions that sustain life. Genetic information for reproduction is stored in DNA or RNA. These ingredients organize in combinations that yield complex structures, which in turn form the basic foundation for all living things. An active area of astrobiology research—with weighty philosophical implications—is whether this pattern of life could be similar or different on other planets. Before we begin to explore other systems, however, it is important to first understand how life originated and evolved here on Earth.
Origins of life
Many conflicting theories about how life began on Earth have gained and lost popularity over time. Today, two hypotheses dominate scientific discourse. The endogenous hypothesis is that life originated on Earth, in places like deep-sea hydrothermal vents. In contrast, the exogenous hypothesis is that organic building blocks of life instead originated in space and were delivered to our planet via comets and asteroids. Both view the origin of life as a gradual, almost inevitable, procession of simpler compounds binding together to form more complex organic molecules.
Approximately 3.8 billion years ago, Earth was almost unrecognizable with its inhospitable lack of atmosphere, scorching temperatures, and violent volcanic activity. In the endogenous model, scientists assume that life began amidst this teeming chaos. In the 1950s, biochemists Stanley Miller and Harold Urey tested this hypothesis and recreated the early-Earth conditions that likely spawned life: a soupy mixture of methane, water, and ammonia were combined with occasional jolts of electricity to simulate lightning. The researchers discovered the spontaneous formation of multiple organic compounds—specifically, amino acids, which are the building blocks of proteins and essential components for life. Scientists hypothesize that these amino acids then self-assembled using pockets in nearby rock formations as a mineral template, eventually leading to the organized structures we see across life today.
Alternatively, in the exogenous model, scientists hypothesize that life originated elsewhere in space and was transferred onto Earth by bombarding comets. This would suggest that life—or at least the precursors to life—is not unique to Earth but is rather a feature of the larger universe. The exogenous model also hypothesizes that water on Earth and other habitable planets was deposited from ice-encrusted comets and that these ubiquitous ice particles were irradiated with cosmic rays for billions of years, forming complex organic structures from simpler elements. Mathies’ group modeled this process in the laboratory and saw, like the Miller-Urey experiment, the spontaneous creation of amino acids. The implications of this simple observation are borderline fantastical: every single habitable planet in the solar system got not only its water from these interstellar particles, but also the inorganic ingredients for life.
Whether life has a single origin within the universe or many, it is thought that life on other planets could be very different than what we have on Earth. It raises the question: what could extraterrestrial life look like?
Life beyond
Life on other planets could be entirely unlike life on Earth due to different environmental conditions, evolutionary adaptations, and random chance. There may still be proteins and other familiar molecules, but they might look and behave very differently from those here on Earth. While there has been much speculation about these differences, little if any concrete data have been produced thus far. However, most scientists agree that the large, animate organisms we’re familiar with—mammals or reptiles, for example—are unlikely to exist on other planets.
“We’re probably dealing with bacteria,” says Mathies. “We know that [some] bacteria survive in very extreme environments.”
These special bacteria are called extremophiles, organisms that not only grow but also thrive in harsh environments that are otherwise lethal to life(see “Toolbox”, page 36). Extremophiles could be predecessors to life on Earth and potentially elsewhere, as they are likely the only organisms that could withstand the inhospitable conditions of early Earth.
Douglas Clark, dean of the chemistry department at UC Berkeley, is studying the extremophiles living at the furthest limits of life. “There are extremophiles that can live in very dry conditions, very high metal or salt concentrations, or very acidic or basic conditions,” says Clark. Some of these extremophiles have been found in deep-sea hydrothermal vents, surviving in temperatures that would melt lead—around 340°C. There are also barophiles, or pressure-loving bacteria that can withstand pressures 250 times greater than that of Earth’s surface, which would crush our unnervingly fragile human bodies well beyond the point of recognition. Psychrophiles are ice-loving microbes, found deep within frozen glaciers and still active at -40°C. There’s even a microbe in the Guinness Book of World Records for being able to tolerate staggeringly large doses of UV radiation. “Pretty much everywhere that people have looked for life on Earth,” says Clark, “they found it.”
The Clark laboratory is recreating the extreme environment of early Earth to examine how bacteria adapt to extreme stress. Understanding the mechanisms by which extremophilic microbes withstand hostile environments has direct implications for life elsewhere within the harsh, seemingly barren cosmos.
Clark is researching the structure and function of proteins from Methanocaldococcus jannaschii, a microbe that was isolated from a deep-sea hydrothermal vent. A number of proteins from M. jannashii are stable at temperatures and pressures that would be instantaneously lethal to many surface dwellers. Characterizing these highly stable compounds has not only direct applications for biotechnology, it is also expanding the way we define life.
Understanding how extremophiles survive in the unlikeliest of habitats widens the number of planets that can be designated as habitable. For example, life on Mars—traditionally considered an inhospitable planet—would have to tolerate an environment with very little water, high concentrations of toxic compounds, and large doses of radiation. By studying extreme organisms on Earth, we can better understand the feasibility of life among the desolate cosmos.
Signs of life
“If you want to look for signs of life,” says Mathies, “we first assume that it is carbon-based life.”
Of all the possible signals that could be used when searching for extraterrestrial life, amino acids—the same organic molecules that spontaneously formed within the Miller-Urey experiment—are the primary target. There are approximately one billion amino acids present in the proteins of a single bacterial cell. “So rather than looking for one bacterium, I look for a billion amino acids,” says Mathies. Looking for this larger signal of amino acids improves the chance of detecting life on other planets. This is critical because if life is not detected, then NASA probes will likely never return to that planet. Amino acids can remain stable for long periods of time, depending on the environment, allowing us to look back in time for biological signatures that were deposited up to tens of thousands of years ago.
Part of the research into probing for extraterrestrial life takes place here on Earth, where ecosystems comparable to those on neighboring habitable planets are studied as a proxy. “If you can’t detect life here in the most extreme conditions, how are you going to do it in space?” says Mathies. The Atacama Desert in Chile is one of the primary testing grounds for life-detecting equipment before space launch. The Atacama is the driest non-polar region in the world, receiving about half an inch of rain per year. Its environment is analogous to the rocky, barren landscapes on Mars.
Mathies’ bioanalytical chemistry research group used the Atacama Desert to test their microfabricated bioanalysis systems, or “lab-on-a-chip” technology, before sending it to the Red Planet. These are portable instruments used for in-place detection of the chemical signals of extinct or existing extraterrestrial life on Mars, Europa, and potentially elsewhere. The instruments work by taking a small soil sample and using a water extractor to isolate biological markers such as amino acids. These isolated markers are then run through a wafer-thin capillary system to separate them by size and thus determine the identity of the biomarkers.
The research team used their Mars Organic Analyzer lab-on-a-chip to successfully detect amino acids from past terrestrial life beneath the harsh and heavily irradiated soil surface in the Atacama. Ground-testing this device in one of the most Mars-like regions on Earth enabled it to pass the clearance for space flight, and it will soon be pummeling through the galaxy towards the Red Planet in July 2020 as part of the European Space Agency’s ExoMars Mission.
The likeliest candidates
Mars, the familiar red orb in our night sky, might not be the best place to search for extraterrestrial life, despite its proximity and presence of water as ice. “It’s probably there,” says Mathies, “but it’s not a good place to look because the samples are hard to get.” The Martian surface, like the Atacama, has been sterilized by harsh radiation for hundreds of thousands of years. If signs of biological life exist, they are likely far beneath the surface in the hard-to-reach, ice-encrusted northern region of the planet. Even a relatively simple tool like a drill would add a lot of additional mass to a spacecraft, which is prohibitively expensive.
Recently, scientists have turned to Enceladus, an icy moon of Saturn. In May 2011, NASA reported that Enceladus “is emerging as the most habitable spot beyond Earth in the solar system for life as we know it.” The moon has a rocky core and a thick surface of ice, but between these layers is an ocean of liquid water. The water here is liquid because of the gravitational tides that come from its wobbling, oval-shaped orbit around Saturn, which generates heat deep within Enceladus from friction. In 1981, the Voyager II spacecraft collected data on a flyby mission that indicated Enceladus was a promising target. Since then, scientists have used satellite images to show the presence of cracks in the ice layer where plumes of water spew into space, also likely caused by the gravitational tides.
The plumes of water from Enceladus are a promising solution to most of the difficulties in searching for extraterrestrial. Not only does Enceladus have liquid water, but that water is erupting into space, making sample collection possible without landing on the moon. These plumes extend up to 300 miles from the surface, with a width of about 30 miles, making them relatively easy for a spacecraft to fly through.
Mathies is currently developing an instrument to probe the plumes of Enceladus for extraterrestrial life. In collaboration with the Space Science Lab at UC Berkeley, the research team is building an instrument called the Enceladus Organic Analyzer. Weighing around five pounds, this device would capture a small amount of icy material in a single pass through a plume of Enceladus, with the intent of detecting amino acids. If the team does not detect amino acids, it could mean one of several things: the device was not sensitive enough to detect the amino acids; there is not (or never was) life on this icy moon; or life on Enceladus is dramatically different from life on Earth. The cost to build this instrument is a whopping $30 million, but that cost pales in comparison to the cost of sending the device to Enceladus, which is expected to be at least half a billion dollars. This enormous amount of time and money will likely result in a single, high-stakes pass through an ice plume, or five to ten passes if the spacecraft went into orbit around the moon. The collected samples would then be instantaneously run through the lab-on-a-chip device, and the results would be digitized and relayed back to Earth via satellites. If the Enceladus Organic Analyzer is successful, its applications on other celestial bodies are innumerable.
Future directions
A growing branch of astrobiology is working to reimagine non-Earth-like life, moving beyond common assumptions about amino acids, carbon, and water. These so-called hypothetical biochemistries include the possibility of non-carbon-based life forms. A likely candidate—although there is currently no evidence to back the hypothesis—is the silicon-based organism. Silicon is the second most common element in Earth’s crust, and we are most familiar with it in the form of sand. Silicon has similar chemical properties to carbon but lacks the versatility of forming as many diverse compounds. However, silicon-based organisms could be biologically feasible in different environments than we experience on Earth.
“It’s hard to imagine a bacterial cell very similar to what we’re used to, using biological building blocks that we’re familiar with, and tolerating much beyond the fairly narrow range of conditions that we have on Earth,” says Clark.
Life on other planets could also be non-cellular, resembling viruses. However, this hypothesis has controversial implications, as many biologists do not consider viruses to be alive, as they require other organisms to replicate.
Given how many potentially habitable planets should statistically be present in the universe, what are the chances that there is life very different from how we know it? “You tell me,” says Clark, “There are a lot of conflicting opinions about it, and that’s the nature of the beast.”
Briana Boaz is a graduate student in integrative biology.
Image credit: Dennis Sun
This article is part of the Fall 2017 issue.