Snuggling up to harmful chemicals in our couches and coats
By Emily Cook
July 26, 2017

Harmful chemicals are part of our daily lives. They exist in anything from pesticides on our food to the plastic of our water bottles. But do we really know the full extent of how these chemicals affect our health over our lifetimes? Unbeknown to most of us, there is a class of emerging contaminants that cause cancer, immune suppression, endocrine (hormone) disruption, elevated cholesterol, and obesity—and you probably have them inside of you right now. You touch them every day in items such as food wrappings, carpet, upholstery, clothing, paints, pesticides, and non-stick cooking ware. These ubiquitous, harmful agents are called per- and polyfluorinated alkyl substances (PFASs).
PFASs came to the public’s attention in the late 1990s when in Parkersburg, West Virginia, a cattle farmer found green, foamy water flowing from a pipe into a stream on his land. His cows were dropping dead, but veterinarians wouldn’t tend to them because the likely source of the water contamination was DuPont, a multibillion-dollar chemical company that had a plant in Parkersburg. DuPont essentially owned the town, so the farmer got no local help for solving this problem. The farmer hired an attorney named Rob Billot who spent the next few years researching DuPont’s chemical products and their health effects. What he found was startling.
DuPont had been manufacturing PFASs since the mid-20th century and was also studying their properties and the health effects the chemicals had on their workers. They discovered in the 1970s that the workers had high concentrations of perfluorooctanoic acid (PFOA), a type of PFAS, in their blood. Over the next two decades, it was discovered that PFOA causes birth defects as well as testicular, pancreatic, and liver cancer—but DuPont did not disclose this information to its workers or to the public.
Despite these facts and observing that the cancer rate of their workers was increasing, DuPont continued to produce these compounds and sell them in products. All the while, PFASs had been contaminating the drinking water supply in Parkersburg, potentially harming thousands of residents.
Billot informed the United States Environmental Protection Agency (EPA) of his findings in 2001 and recently settled 3,500 personal injury suits against DuPont. DuPont and its daughter company, Chemours Co., agreed to pay $671 million to settle these suits. The EPA is now heavily involved in researching the prevalence of PFASs, while also establishing the health advisory limits for PFASs.
Currently, out of the many different types of PFASs, only two are regulated at water treatment plants—perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). The limits are set at 70 parts per trillion, or 70 nanograms per milliliter. These values have to be set so low because PFASs are able to accumulate in our bodies over time. Unfortunately, recent EPA research has found concentrations much higher than 70 nanograms of PFOS and PFOA in various rivers, drinking water sources, and fast food packaging.
Since treatment plants are required to meet the limits, enforcing regulations such as these encourages research into effective and inexpensive removal techniques. In order to fully understand why it is important to remove emerging contaminants such as PFASs, we must know what PFASs are and why they are so harmful.
PFAS is a broad term for a long carbon chain that has carbon-fluorine (C-F) bonds instead of the carbon-hydrogen bonds found in the better-known class of chemicals called hydrocarbons, which include fuels such as butane and propane, among others. The C-F bond is one of the shortest and strongest chemical bonds, making PFASs very stable and long-lasting compounds—a problem when removing these compounds from contaminated water and nature.
[caption id="attachment\\_14812" align="alignleft" width="476"][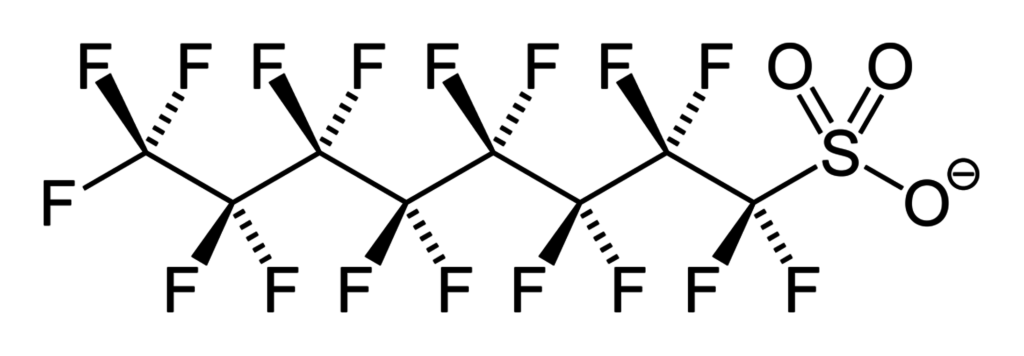](http://berkeleysciencereview.com/?attachment\_id=14812) Chemical structure of perfluorosulfonate (PFOS), a type of PFAS.[/caption]
In addition to being strong, the C-F “tails” of these compounds is extremely hydrophobic and oleophobic—that is, they don’t dissolve well in water or oil. As a result, these compounds repel both oil and water, making them very good ingredients for rain coats, tents, and stain-resistant fabrics.
There are many different types of PFASs because there can be different C-F tail lengths. These shorter or longer variations can have slightly different properties. For example, a PFAS with 6 carbon and 11 fluorine atoms will behave differently than a PFAS with 8 carbon and 15 fluorine atoms—the fewer the carbons, the harder they are to remove from the environment.
PFASs can get stuck in our body and accumulate over time. Once consumed, longer PFASs can remain in our bodies for months or even years, and this accumulation is one of the reasons PFASs are such a danger to humans and other creatures. Their stability also makes them “travelling chemicals”, meaning they can be transported via water, attached to solids, or even partitioned onto airborne particles. Because they travel and do not break down, PFASs are geographically ubiquitous: they are found across the globe, even in Antarctica.
[caption id="attachment\\_14814" align="alignright" width="428"][](http://berkeleysciencereview.com/?attachment\_id=14814) Chemical structure of perfluorooctanoic acid (PFOA), a type of PFAS.[/caption]
Most conventional wastewater treatment plants currently do not effectively remove PFASs from the water, even though they are required to remove PFOA and PFOS down to 70 parts per trillion. Thus, there are many research efforts looking into removal strategies for PFASs at treatment plants. One promising method is adsorption—the “sticking” of PFASs to solid compounds. If treatment plants can succeed in making PFASs adsorb to materials, the PFASs can be settled out (removed by letting the PFAS-adsorbed solids sink to the bottom of a reactor) of the water.
Adsorption to materials such as silica, alumina, and activated carbon has proven effective, but the efficiency depends on the material. The most promising material seems to be granular activated carbon (GAC), which is cheap and effective, but would require frequent replacement at treatment plants.
There are also research efforts underway to break down PFASs into different, less harmful compounds. Chemical oxidation is the process of using an oxidant (such as persulfate or ozone) to attack and break chemical bonds. The goal of chemical oxidation of PFASs is to break down these compounds enough to be short, harmless hydrocarbon chains without fluorinated carbons. This process is still imperfect—effective oxidation requires very high temperatures and acidic conditions, which can be harmful in the environment, where chemical oxidation is hoped to be implemented. In addition, oxidation cannot break down some types of PFASs.
Although there are some promising removal techniques, research on PFASs is still very much in its infancy. We don’t fully understand how all the different PFASs are affecting our health and the environment, yet PFASs continue to be commonly used in industry.
PFASs are just one example of a harmful chemical we come into contact with every day. In order to be a more healthy and sustainable society, we must reject the use of these sorts of harmful compounds in industry and instead encourage the search for and implementation of safer options. Until we reach that point, though, we should be grateful to the observant farmers and dedicated attorneys among us.
Featured image credit: By Caleb Wilkerson via Flickr.