 Nanoparticles of zinc oxide. Source: Claire Kunkle
Nanoparticles of zinc oxide. Source: Claire Kunkle
California is in the midst of a historic drought that impacts both agriculture and residential water supply in unprecedented ways. Water—particularly freshwater—is a precious resource that is rapidly being depleted while extreme weather exacerbates drought. These harsh conditions come at a time when the swelling population demands more water for health, hygiene, and less intuitively, for electricity.
Around 41 percent of freshwater in the United States is used solely for generating electricity, primarily as a coolant. That’s far more water than the demands of irrigation farming. It’s easy to understand why when you consider that one thermoelectric power plant can easily draw in a staggering one billion gallons of water per day.
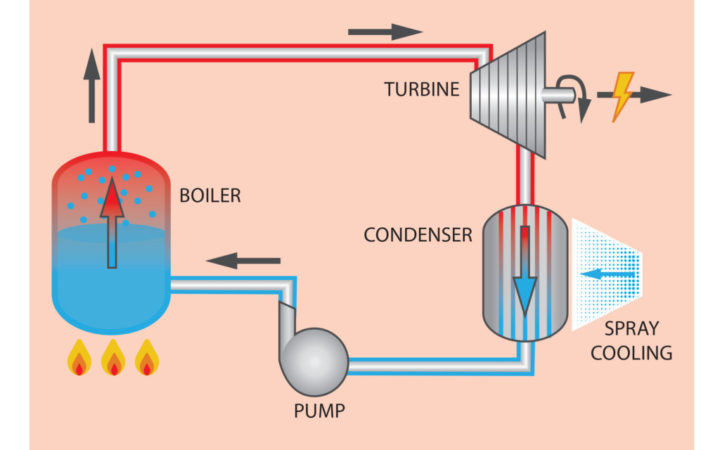 The Rankine Cycle, where water is boiled to create steam and run a turbine to create electricity. Source: Holly Williams
The Rankine Cycle, where water is boiled to create steam and run a turbine to create electricity. Source: Holly Williams
Why do power plants require so much freshwater to function? In a standard power plant, electricity is derived by generating and recondensing steam. The steam is used to spin a turbine, which, in turn, powers an electric generator. Once the steam has gone through the turbine, it must be cooled back down to a liquid. There are many ways to condense steam back to a liquid, but the most common is to feed it through many small pipes that are cooled from the outside. Often, this is done through spray cooling, a process that involves water—lots of it.
In spray cooling, water is sprayed onto the outside of steam-filled pipes. The water heats up as it is in contact with the hot pipe and eventually evaporates, removing heat from the steam inside the pipes. In order for this process to work well, the water should remain on the pipe’s surface until it evaporates. A primary objective is to prevent droplets from running off before they can absorb the heat from the pipe and evaporate.
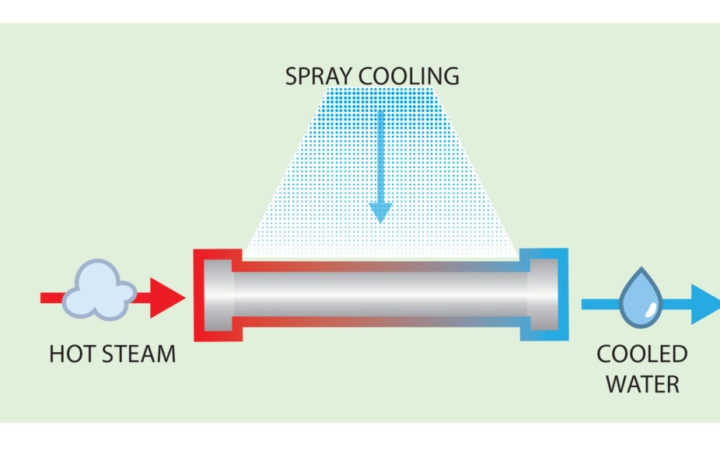 Process of Spray Cooling to condense water from vapor to liquid; first cooling water spreads on the hot pipe surface, then, it evaporates and takes heat as it rises. Source: Holly Williams
Process of Spray Cooling to condense water from vapor to liquid; first cooling water spreads on the hot pipe surface, then, it evaporates and takes heat as it rises. Source: Holly Williams
One way to prevent this waste of water is to put a coating on the pipe that makes water stick and spread on the pipe’s surface. At UC Berkeley, Professor Van Carey’s lab is investigating just how to design surfaces to make water stick. On plain metal pipes, water beads up and can bounce, drip, or roll off when water is sprayed on them. However, when nanoparticles of zinc oxide are grown to form a very thin coating on the pipe, water droplets can stick and spread out. The more the droplets spread out, the less likely they will be to bounce off and be lost. Longer contact with water droplets means better heat transfer—cooling off the pipes, and thus the steam inside them.
To actually manufacture these surfaces, nanoparticles are deposited on a polished copper surface that is then baked in a growth solution for upwards of 12 hours. This growth solution is mostly water with dissolved substances that encourage the growth of nanoparticles. During baking, the nanoparticles grow into pillars. The process is similar to growing sugar crystals by sticking a string into sugar water, though the crystals are on a much smaller scale. Each of these zinc oxide pillars is about 25 times thinner than a human hair.
Specialized microscopes allow for observation of the intricate nano-pillars all over the pipe’s surface. The more numerous the pillars, the rougher the surface, and the more places for water to seep in and stick rather than roll off. The science behind this is all related to physics and surface tension. If you put a drop of water onto a piece of plastic versus a piece of Velcro, you will find that the Velcro holds the water in place, by catching it between all the little fibers. The nano-structure coated surfaces act like nano-Velcro for water.
The application of surfaces like this is not simply a pipe dream. Currently, a multimillion dollar grant (CERC-WET) is funding a collaboration between the US Department of Energy and the Chinese Energy and Water research groups to reduce water footprints worldwide. In the future, sticky pipes may be just what we need to keep our water from slipping from our grasp.
This article is part of the Fall 2016 issue.



