 Credit: Florian Brown-Altvater
Credit: Florian Brown-Altvater
Chemists from UC Berkeley wondered if they could take a metal used to speed up chemical reactions in the lab and get it to do the same thing—or maybe even something new—within a protein. By adding in this metal, they engineered a protein with special abilities not found in any naturally occurring protein.
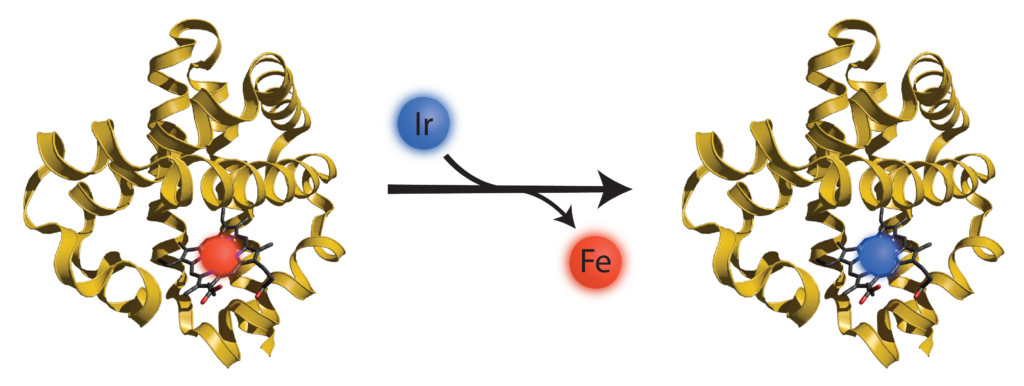
Enzymes are the proteins nature uses to speed up, or catalyze, chemical reactions so they occur in nanoseconds. Many important enzymes combine proteins with a metal atom to form a metalloenzyme, and the type of metal they contain allows them to bind specific molecules. Hemoglobin, for example, is a metalloprotein that uses iron to carry oxygen through the bloodstream from our lungs to our tissues. A related protein, myoglobin, uses iron to store oxygen in muscles. Professor John Hartwig’s lab in the Department of Chemistry at UC Berkeley replaced the iron in myoglobin with iridium, a precious metal that is not naturally found in the human body. With iridium swapped in, the bionic enzyme now turned carbon-hydrogen bonds to carbon-carbon bonds.
These new proteins are of great interest to chemical manufacturers, and could be used to make chemicals in the products we use daily, like biomedical drugs, fuel, and agrochemicals. “The overall goal is to make a process more efficient,” explains Hanna Key, a graduate student in Hartwig’s lab and co-first author of the work. “In general, catalysis is about greener chemistry: less waste and more savings of time, money, and crude materials.” By engineering enzymes that reduce the number of steps a reaction takes, we reduce time and waste, and the costs to our pocketbooks and the environment are reduced, too.
This article is part of the Fall 2016 issue.



