
Bottles of vibrantly colored chemicals line dimly lit shelves. On a bench below, a Bunsen burner flickers beneath a flask of deep red liquid, illuminating the face of a scientist perched on a stool nearby. The scientist swirls a glowing, neon blue liquid, scrawling observations in a notebook.
The movie ends. You return to reality. In our nonfiction world, the substances that decorate many laboratories—and the chemicals of everyday life—are most often colorless. The rare colorful materials are the paints on nature’s palette. Plants contain thousands of different chemicals, but it only takes a single substance—chlorophyll—to make a leaf green.
In many cases, the colors we encounter in the world come from pigments like chlorophyll, which absorbs light of some wavelengths and reflects other light. The mix of wavelengths of the reflected light, which depends on the chemical properties of the pigment, determines the color we perceive the light to be. Given the chemical diversity of substances, this may sound like a satisfying explanation of our colorful world.
But there are many other ways to generate color, and humans and nature alike have capitalized on them. Research on colorful phenomena has inspired many technologies, from sensors to drug treatments, and has provided a glimpse into nature’s inner workings, from evolution to quantum physics.
The hidden forest in a butterfly’s wing
In Professor Nipam Patel’s office in his lab at UC Berkeley, drawers are filled with butterfly wings in captivating colors and a variety of patterns. This makes the wings pleasing to the naked eye, but gaining a true appreciation of them requires examination at the nanoscale.
 The nanoscale architecture of some butterflies' wings creates vibrant colors. The scales of blue morpho (left) and buckeye (center) butterflies are layered and patterned with nanostructures made of chitin that interact with light, cancelling out some colors and amplifying others. For the emerald swallowtail (right), a combination of nanostructures—one that reflects yellow light and another that reflects blue—leads to a brilliant green color.
The nanoscale architecture of some butterflies' wings creates vibrant colors. The scales of blue morpho (left) and buckeye (center) butterflies are layered and patterned with nanostructures made of chitin that interact with light, cancelling out some colors and amplifying others. For the emerald swallowtail (right), a combination of nanostructures—one that reflects yellow light and another that reflects blue—leads to a brilliant green color.
At a glance, blue morphos’ wings are a shimmery blue, but viewing them at high magnification with an electron microscope reveals that they’re textured with microscopic layers of tree-like structures made of a material called chitin. But chitin isn’t blue—in fact, it isn’t a pigment at all. When light hits a blue morpho wing’s surface, the branches of these structures obstruct the path of the incoming light, which bends around the branches the way water in a stream bends around a rock, scattering in the wing’s forest. The scattered photons then interact with each other, producing a spectacularly rich blue hue.
This interaction is called interference: when waves of light interfere with one another, color can be intensified or reduced. If the crests of a light wave align with the crests of another wave of the same wavelength, the waves are said to be in phase. In this case, the interference is constructive; like waves gathering on the ocean, a larger wave is formed, and the light of that wavelength is intensified. If the opposite happens, and the crests of the waves don’t align, the intensity of light of that wavelength is dampened.
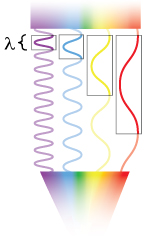 When light traveling through air hits a thin film, some of the light is reflected and some is transmitted into the film. Once the light reaches the bottom of the film, some light will be reflected back again to the surface of the film. In case a, the crests of the waves are aligned (in phase), which amplifies the light of that color. In case b, the crests of the waves are not aligned (out of phase), which dampens light of that color. The thickness and optical properties of the material through which the light travels determines the colors of light that will be amplified. Illustration: Ashley Truxal.
When light traveling through air hits a thin film, some of the light is reflected and some is transmitted into the film. Once the light reaches the bottom of the film, some light will be reflected back again to the surface of the film. In case a, the crests of the waves are aligned (in phase), which amplifies the light of that color. In case b, the crests of the waves are not aligned (out of phase), which dampens light of that color. The thickness and optical properties of the material through which the light travels determines the colors of light that will be amplified. Illustration: Ashley Truxal.
Rachel Thayer, another graduate student in the Patel lab, is using yet another structurally colored butterfly, called the buckeye, to determine which genes are involved in color generation. To find them, she mates butterflies that are bright blue to drab brown butterflies, yielding a generation with intermediate colors.
Thayer then mates these intermediate offspring to one another, and the resulting progeny are a jumble of different colors: some are blue, some are brown, and many are in between, with patchwork brown and blue scales or even different colors altogether. She can then look for the genetic differences among these different classes of butterflies, revealing which areas of the genome contain information responsible for building the nanostructures that give the butterflies their color. Finding the genes is a work in progress, but once they’re tracked down, researchers could investigate them further, one at a time, to see how they work in concert to produce these minute structures.
Over and over, nature has invented this and other types of structural color; it has arisen independently in many other butterfly species, along with peacocks and several beetle species. Creating synthetic versions of these structures has so far generally proven prohibitively expensive—but if better manufacturing techniques are developed, there are many possible applications.
By incorporating nanostructures that shift color as the viewing angle changes, currency could be made more difficult to counterfeit. Paints with colors based on structure rather than pigments might be more stable when exposed to light and thus better able to stand the test of time. And since the type of structural coloration in the blue morpho is so strongly dependent on the properties of the environment around it, it might be possible to design sensors based on the butterflies’ wings that are very sensitive to dangerous chemicals in the air.
Blind bacteria develop colorful adaptations
Butterflies and birds use color for a variety of purposes, such as camouflage and signaling that they would make good mates. But for some organisms, color is a byproduct of other vital processes. Cyanobacteria, for instance, produce orange carotenoid protein (OCP)—a color-changing defense system that protects them from excessive light exposure. Although these organisms need light for photosynthesis, too much light can cause damage to critical biomolecules. By interacting with other proteins, OCP allows the bacteria to dissipate the energy harmlessly as heat, changing from orange to red in the process.
OCP’s orange hue arises from one of the better-known types of color generation. The protein contains a pigment, called a carotenoid, which selectively absorbs some wavelengths of light while reflecting others. But the mechanism behind its color change has only recently been revealed.
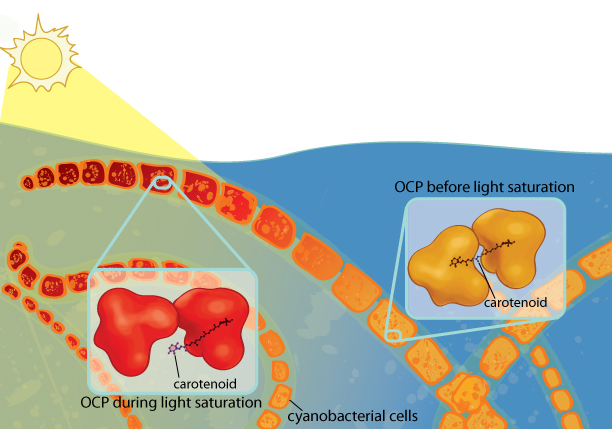 Cyanobacteria need light for photosynthesis, but too much light can damage them. Orange carotenoid protein (OCP) protects the cyanobacteria from excess light. When OCP absorbs light, it changes conformation, causing the carotenoid (a pigment) it contains to migrate to a new location in the protein. As a result, the carotenoid changes from orange to red, a phenomenon that can be seen with the naked eye. Illustration: Ashley Truxal.
Cyanobacteria need light for photosynthesis, but too much light can damage them. Orange carotenoid protein (OCP) protects the cyanobacteria from excess light. When OCP absorbs light, it changes conformation, causing the carotenoid (a pigment) it contains to migrate to a new location in the protein. As a result, the carotenoid changes from orange to red, a phenomenon that can be seen with the naked eye. Illustration: Ashley Truxal.
According to research by Cheryl Kerfeld’s group at Lawrence Berkeley National Laboratory, when too much light enters the cyanobacterium, the carotenoid pigment absorbs the excess light, which provides the energy to break a hydrogen bond. This causes the protein to open up to the water-filled environment in the cell, exposing the carotenoid within the protein to water molecules. The carotenoid is hydrophobic, like oil, so it burrows deeper into one side of the protein, away from the water.
Since the configuration of the carotenoid has changed, it begins to absorb a different part of the spectrum of light, causing it to become red instead of orange. And importantly for the bacterium, the part of the protein the carotenoid used to be affixed to is no longer buried. The newly-revealed surface of OCP then latches on to a light-harvesting protein complex called the antenna, allowing OCP to dissipate the potentially harmful excess light energy.
Since the Kerfeld group’s recent discovery of this mechanism, new avenues of applied research have already been spawned. “Once you understand how something works, you have the ability to tinker with it and to tune it,” Kerfeld says. Although there are other photoprotective proteins in nature, some of OCP’s other properties make its use particularly promising. Despite the fact that the carotenoid pigment in OCP is hydrophobic, the protein on the whole is hydrophilic, making it easy to work with for applications like bio-based solar panels.
Water solubility also means the protein will likely behave well within cells, opening up a whole range of other uses. “Maybe we could use that shape change to toggle some other kind of switch,” Kerfeld says, such as a control mechanism for drug delivery. This type of shape-shifting could make it possible to delicately orchestrate the location and timing of drug release with light. For humans, this would be particularly useful in the skin, where light can easily penetrate. For instance, a person could take a drug orally, but have the drug only become active when it reaches skin exposed to the right wavelength of light.
The Kerfeld group is currently in the early stages of researching how this could be achieved. “What we’re trying to do is design a switch such that when you give it a specific type of light, the protein opens up, changes shape and exposes a site that was previously hidden in the orange form of the protein,” Kerfeld says. The newly-revealed site on the protein could be engineered to bind another molecule in the cell, triggering an effect the light alone wouldn’t normally have, such as releasing the drug in the skin.
Although the light-activated rearrangement of OCP is crucial for protecting the cyanobacteria from dangerous excess light, the color change itself may not be important to the bacteria: it could just be a symptom of absorbing light. But to human observers, the change from orange to red signals that the protein is active and draining energy from light. Kerfeld explains, “The cool thing about OCP is when it’s actually absorbed light, you can really see the difference,” so specialized equipment isn’t even needed to detect the change. So while the bacteria may be indifferent to the color change, it could be used by humans as a visible marker indicating whether the protein is on or off—a useful readout for whether it is performing the intended function.
Quantum color
Pigment-based color like the color of the carotenoid in OCP and, to a lesser extent, structural color like that found in some butterfly wings, are some of the more common types of color on Earth. But colors can also be produced by other phenomena—and the science behind one of them is stranger than fiction.
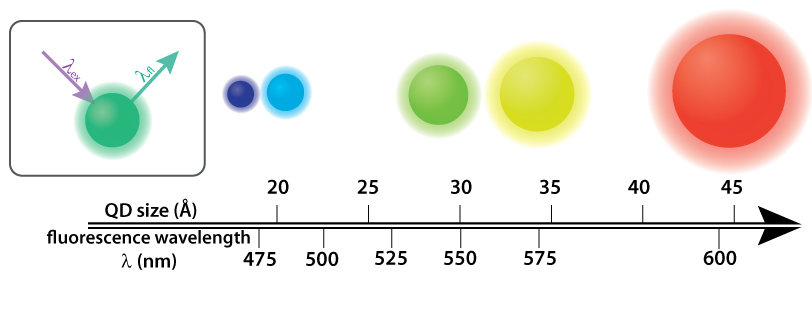 Quantum dots (QDs) are semiconductor nanocrystals that fluoresce in a variety of colors determined by their size. Above, cadmium selenide (CdSe) QDs sit atop a single axis that indicates both their diameter and the color they fluoresce. Inset: In order to give off light by fluorescence (fl), the QDs require higher energy light, such as UV light, (ex) to be shined on them. Infographic: Ashley Truxal; Infographic data: DOI: 10.1021/cm034081k.
Quantum dots (QDs) are semiconductor nanocrystals that fluoresce in a variety of colors determined by their size. Above, cadmium selenide (CdSe) QDs sit atop a single axis that indicates both their diameter and the color they fluoresce. Inset: In order to give off light by fluorescence (fl), the QDs require higher energy light, such as UV light, (ex) to be shined on them. Infographic: Ashley Truxal; Infographic data: DOI: 10.1021/cm034081k.
In Professor Paul Alivisatos’s lab in UC Berkeley’s Department of Chemistry, the shelves aren’t as visually bland as the ones in many labs—in this lab, making colorful materials is routine. The group studies nanoparticles, tiny clusters of atoms just billionths of a meter across in at least one dimension. Particles like these are unique because the way they interact with light is exquisitely sensitive to their size.
When a substance absorbs light, the electrons within it can become excited—that is, the electrons can move to a higher-energy state. In most cases, the electrons relax back down to their normal state, called the ground state, without causing too much commotion. The light that’s reflected back instead of being absorbed gives the substance its color. This is the mechanism behind the color of pigments like the carotenoid in OCP.
But in some cases, the excited electrons transition to a state that allows them to emit light as they fall back down to the ground state, a process known as fluorescence. “Any material that can absorb light can also emit light in the form of fluorescence,” explains Natalie Gibson, a graduate student in Professor Stephen Leone’s group in the Department of Chemistry at UC Berkeley who collaborates with the Alivisatos lab. Although nanoparticles aren’t the only materials that can fluoresce, the way they fluoresce is special.
Gibson studies quantum dots, a type of nanoparticle that glows intensely. Looking at a solution containing quantum dots, the fluorescent color is obvious and constant. But individual quantum dots don’t continuously fluoresce—they flicker from higher to lower fluorescence over time, a phenomenon known as quantum dot blinking. There are many proposals to explain the mechanism behind this blinking phenomenon, and Gibson has focused her research on understanding the process. Recently, she’s steered her work toward unconventional nanoparticles (called perovskite nanocrystals) with less-understood properties. The states responsible for causing blinking may be very different in perovskite nanocrystals than in more traditional quantum dots. According to Gibson, “We have gained a lot of knowledge about [traditional] quantum dots over the last fifteen or twenty years, so it will be interesting to see if the same kind of dynamics apply to perovskite quantum dots or if we are dealing with different physics.”
Beyond being interesting from a purely physical perspective, quantum dots have many budding applications, and understanding their physical properties will only make them more useful. For one, quantum dots produce colors that can be tuned by their size. Smaller quantum dots produce bluer light, while larger ones fluoresce red, with the sizes in between representing all the colors in the visible spectrum. In addition to being tunable, quantum dots are also easy to synthesize, making them promising for commercial applications. Liquid crystal displays (LCDs), commonly used as TV screens, require illumination from a light source—for example, quantum dots—to produce images. Today, most LCDs are backlit by light-emitting diodes (LEDs), but typical LEDs produce light in a broader wavelength range than that of quantum dots. The narrower wavelength range emitted by quantum dots results in less light being generated in unwanted wavelength ranges, making it easy to produce vibrant colors with precision.
Although quantum dots have the potential to produce light that is brighter and with more precise wavelength ranges than standard LEDs, current displays using quantum dots still rely on LEDs to generate some of the light (and to excite the quantum dots in the first place). But the technology is already proving successful—quantum dots have been incorporated into displays by a variety of forward-thinking companies, including one co-founded by Alivisatos himself.
More than meets the eye
The applications made possible by our understanding of color in nanoparticles don’t end at the purely practical. In fact, some of their most fascinating uses are artistic.
Nanoparticles’ visual allure was recognized by Bay Area artist Kate Nichols, who has taken up a position as artist-in-residence with the Alivisatos group. She was inspired to work with nanoparticles after hearing features about them on NPR and by a math class in college that introduced her to structural color in the blue morpho butterfly. Nanoparticles’ unique relationship with light is what drew her in.
 Kate Nichols' art exemplifies the beguiling optical behavior of nanoscale matter without screen or scope, using only the naked eye. Only by virtue of their sheer number are these particles visible: trillions of sub-wavelength particles are pressed between panes of glass. This piece: "Figment 1." Silver nanoparticle paint on glass. 22 x 18 inches, 2014. Photo: Donald Felton.
Kate Nichols' art exemplifies the beguiling optical behavior of nanoscale matter without screen or scope, using only the naked eye. Only by virtue of their sheer number are these particles visible: trillions of sub-wavelength particles are pressed between panes of glass. This piece: "Figment 1." Silver nanoparticle paint on glass. 22 x 18 inches, 2014. Photo: Donald Felton.
Nichols’s work "Through the Looking Glass 1" exemplifies the visual difference between nanoparticles and larger particles of the same materials. In the piece, silver nanoparticles sandwiched between two panes of glass form a ghostly purple-blue veil over wispy layers that are the color of ocher—colors Nichols conjured up by controlling the nanoparticles’ size during synthesis. Any silver particles larger than nanoparticles might still be beautiful, but they would all be the same shiny gray.
Working with these materials to develop art, Nichols has mused about the very meaning of color. “We’re used to thinking about light being absorbed or reflected by materials, but light’s also scattered by and transmitted through materials,” Nichols says. As a viewer moves around some of her nanoparticle-based art pieces, or if a dark panel is inserted behind the nanoparticles that screens out transmitted light to leave only the reflected light, the object’s color profile—its relationship with light—can be experienced in different ways.
 Kate Nichols' "Through the Looking Glass 1." Silver nanoparticles on glass. 24 x 45 inches, 2011. Photo: Donald Felton.
Kate Nichols' "Through the Looking Glass 1." Silver nanoparticles on glass. 24 x 45 inches, 2011. Photo: Donald Felton.
“When color becomes a less solid thing, when the color is changeable, the question ‘what color is this?’ breaks down. Instead, we have to ask: what is color?” says Nichols. “And I would say color is really about something’s relationship with light. Nanoparticles have a complicated relationship with light.” Combined with the artist’s design, those properties give Nichols’s nanoparticle-based art its visual intrigue.
Although she now spends more time in her own studio in San Francisco and teaching at the San Francisco Art Institute, Nichols is still working with nanoparticles, returning to Alivisatos’s lab to synthesize them. Through her experience working with the lab, she has gained a new appreciation for color and what it means. “A lot of the time,” Nichols says, “as we go about the world, we sort of take these things for granted.”
This article is part of the Fall 2016 issue.



