
Blood has long been a key ingredient in fantastic tales of eternal youth. Medieval doctors practiced bloodletting to void the body of “evil” impurities, and immortal, blood-sucking vampires have dominated legends for centuries. While these stories now provide plot lines for Hollywood, they also inspire cutting-edge science. By examining the chemical makeup of young blood, scientists at UC Berkeley have discovered a drug that could turn back the age clock.
The revelation began a decade ago when Irina Conboy, now a professor of bioengineering at UC Berkeley, revived an old blood-sharing technique to study aging in mice. Researchers in Conboy’s lab surgically opened the hindlimbs of two mice, one young and one old, and then sewed the animals together, effectively conjoining their circulatory systems. Within several days the young mouse’s blood had begun rejuvenating aged tissues in the old mouse. “It was clear that there’s something in blood that changes with age,” said Conboy. “Then the question became: what could it be?”
Blood distributes an enormous variety of compounds throughout the body. Once blood reaches an organ, life-sustaining oxygen, nutrients, and hormones diffuse into the tissues, saturating the fluid-like environment surrounding individual cells. As molecules arrive, they latch onto receptor proteins that coat the outside of cells. In a process called cell signaling, these receptors act like antennas by transmitting information to the cell’s internal machinery and influencing its behavior. For example, when a person is frightened, their breathing quickens, digestion slows, and muscles tense, preparing to flee or fight for survival. In this instance, the body is reacting to an influx of adrenaline molecules traveling through the blood and targeting receptors in the lungs, gut, and muscles.
Just as adrenaline is secreted to confront danger, other signaling molecules flood the bloodstream when we are angry, in love, or simply getting older. Given the control that cell signaling molecules exert on their targets, Conboy and her team hypothesized that they likely play an active role in the aging process. Hungry for answers, Conboy fostered an ongoing collaboration with the lab of UC Berkeley bioengineering professor David Schaffer.
In order to identify molecules involved in the aging process, the two labs examined changes in the environments surrounding adult stem cells, a cell type known for its role in growth and regeneration. As the body ages and breaks down, adult stem cells stave off aging by replenishing depleted cells and regenerating damaged tissues. Two unique facets of adult stem cells allow them to do this. First, they are undifferentiated, meaning they can divide to create any cell in the body. Second, they are capable of copying themselves indefinitely. Despite getting older, these cells preserve their youth by remaining relatively inactive, which minimizes the internal damage caused by the cells doing work and generating waste.
Conboy noted that a molecule called transforming growth factor-beta 1 (TGF-β1) affected the activity levels of stem cells. In youth, TGF-β1 exists in low levels, preventing unrestrained proliferation of cells and suppressing inflammation. However, as the organism ages, levels of TGF-β1 begin to rise, suppressing stem cell activity. This keeps stem cells from performing their regenerative duties, eventually wreaking havoc on parts of the body, especially muscle and brain tissue.
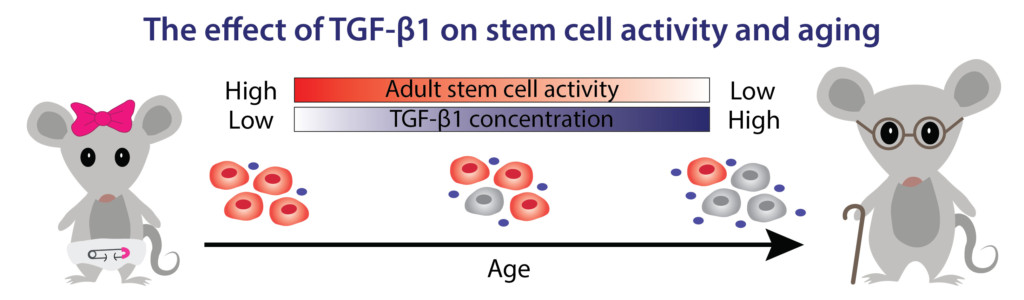 Transforming growth factor beta 1 (TGF-fl1) is necessary for preventing excessive cell proliferation that could otherwise lead to cancer and inflammation. As animals age, the concentration of TGF-fl1 increases, causing stem cells to deactivate and preventing them from adequately responding to tissue and organ injuries. Scientists are investigating how a new drug may lower levels of TGF-fl1 in older animals, allowing stem cells to reactivate and regenerate unhealthy tissues.
Transforming growth factor beta 1 (TGF-fl1) is necessary for preventing excessive cell proliferation that could otherwise lead to cancer and inflammation. As animals age, the concentration of TGF-fl1 increases, causing stem cells to deactivate and preventing them from adequately responding to tissue and organ injuries. Scientists are investigating how a new drug may lower levels of TGF-fl1 in older animals, allowing stem cells to reactivate and regenerate unhealthy tissues.
After isolating TGF-β1 as a culprit, finding a treatment became paramount. Hanadie Yousef, currently a postdoctoral fellow at Stanford and a recent graduate of the Schaffer lab, spearheaded the project to hunt down a molecule that affects the amount of TGF-β1 in the bloodstream. As Schaffer explains, “If it’s a single mechanism that affects multiple tissues, then you can potentially use a single drug to hit multiple birds with one stone.”
Last year, the two teams published research in which they used a drug called Alk-5 kinase inhibitor to decrease levels of TGF-β1 in the bloodstream. Since TGF-β1 prolongs stem cell dormancy, reducing the levels of this molecule amounts to increasing stem cell activity. After several days of administering the drug to aged mice via injection, their adult stem cells began behaving youthfully, repairing injuries in muscle tissues and regenerating tissue in the hippocampus, a region of the brain critical for memory formation.
Fortunately, the drug Alk-5 kinase inhibitor has already safely undergone clinical trials for use against small tumors in humans, and might be quickly implemented in clinical treatments for aging. Given these encouraging results, the two labs are considering ways to evaluate the drug’s efficacy in humans. “The first patients to receive the treatment would be those with traumatic injuries of the brain, muscle, or bone,” said Michael Conboy, a researcher at the QB3 institute in Berkeley. “Treatment will prevent inflammation and allow people to regain mobility faster.”
While an anti-aging pill eludes researchers, some life preservation treatments have become more reality than fiction. As medicine allows us to live longer, should our primary concern be merely increasing lifespan, or striving to enhance “well-span” by remaining mentally and physically capable until our time is up? “I’d be thrilled if I could ski until I’m 90 years old, if that is my lifespan,” Schaffer said. “A high quality of life up until that point is what I’m really working towards.” With this question in mind, researchers press onward, arming themselves with knowledge in the bloody battle against aging.
Dawn Lipscomb is a graduate student in biophysics.
This article is part of the Spring 2016 issue.



