The destruction starts with a modification in the instructions that tell a single brain cell how to grow. As the cell rapidly multiplies into thousands of cancerous cells, an irregularly shaped tumor mass forms, with tendrils that invade deeper into healthy brain tissue. The tumor’s abnormal growth makes it hungry for more oxygen and nutrients, so it hijacks the circulatory system and forces new blood vessels to grow around it. It is resistant to radiation, and is protected by the brain-blood barrier—a series of defenses within blood vessel walls that protect the brain from toxic molecules, but also keep out cancer drugs that could kill the cancerous cells. Left to continue growing, it eventually destroys its host. This is the brain cancer glioblastoma multiforme.
Although it is difficult to fight, glioblastomaalso has a weakness. In its rush to feed itself, it accelerates the blood vessel formation process and creates hole-riddled blood vessels around the tumor. Because cancer drugs are small enough to slip through these holes, they can exploit this defect—but they also need a strategy to cross the blood-brain barrier in order to reach the tumor.
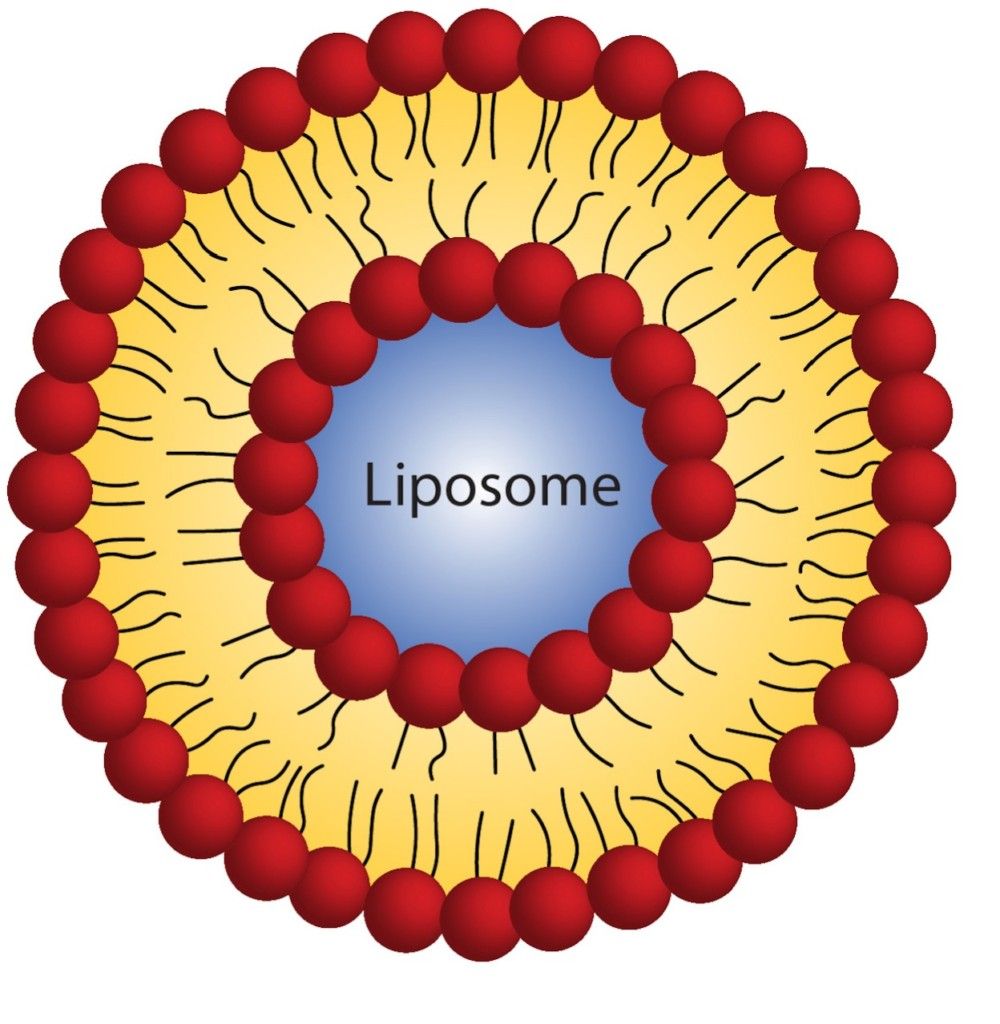
To help cancer drugs access the tumor, Ting Xu, professor of materials science at UC Berkeley, and her collaborators are making tiny particles called nanocarriers that protect the drugs during their journey to the tumor. They recently demonstrated that their nanocarrier, called a 3-helix micelle (3HM), is twice as effective at reaching glioblastoma cells as a liposome, a nanocarrier that is currently the gold standard in drug-delivering nanotechnology.
Scientists make nanocarriers out of biological molecules, such as fatty acids and proteins, and engineer these particles to be effective delivery systems to a tumor. Nanocarriers are designed to be small so they can transport cancer drugs through the holes in the tumor’s blood vessels. They are so small that 1,000 nanocarriers can be lined up around a strand of human hair. To help the nanocarriers identify tumor cells, scientists attach molecules to the surface of nanocarriers that fit into proteins only found on the surface of tumor cells, like a key fits into a lock.
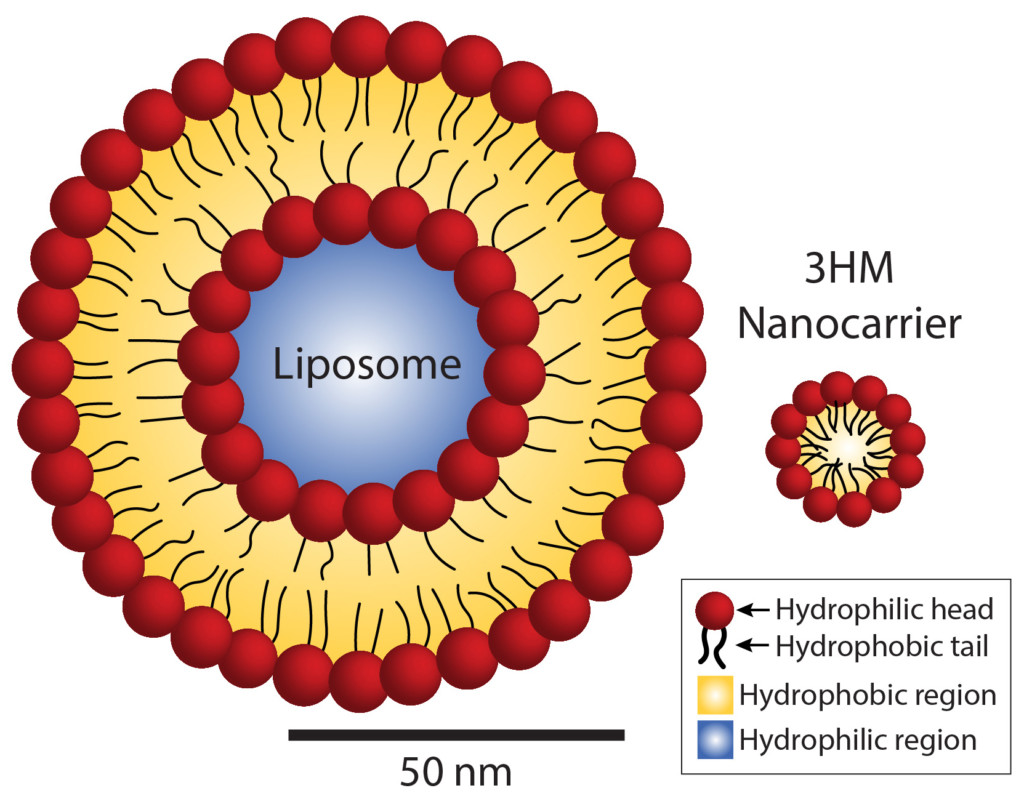 Size comparison of liposomes and 3HM nanocarriers. Whereas liposomes contain two layers of fat molecules, 3HM nanocarriers contain only one layer, allowing them to be much smaller. This compact size makes them more advantageous as drug carriers for cancer therapy.
Size comparison of liposomes and 3HM nanocarriers. Whereas liposomes contain two layers of fat molecules, 3HM nanocarriers contain only one layer, allowing them to be much smaller. This compact size makes them more advantageous as drug carriers for cancer therapy.
When scientists hide cancer-fighting drugs within, nanocarriers act like a horde of miniature Trojan horses. They move through the blood, protecting their drug cargo against attacks from blood compounds that could react with the drugs and render them ineffective. Then, once they leave the blood and enter the tumor tissue, the nanocarriers present their surface attachments to gain access into the tumor cells. At this point, the nanocarriers release their tumor-killing drugs.
A key feature of effective tumor drugs is their ability to penetrate tumor cell walls. Because smaller nanocarriers are more effective at penetrating the tumor, Xu’s group focused on making 3HM smaller than the standard liposomes, and they succeeded—3HM is five times smaller than liposomes. In order to accomplish this, they had to do a bit of molecular origami.
Both liposomes and 3HM are made up of molecules that have water-loving heads and water-fearing tails. In liposomes, these molecules are the same fat-based compounds that make up cell membranes. When dropped in water, two layers of these fat molecules assemble into spheres, with the water-loving heads sticking out towards the surface and the inside of the sphere, and the water-fearing tails pointing inside towards each other.
The molecules that form 3HM, on the other hand, have heads made up of proteins. When placed in water, these molecules assemble into balls, with the tails nestled safely near the ball’s center and the heads sticking out on the surface. Because 3HM has only one layer of these molecules, compared with the liposomes’ two layers, they are much smaller than liposomes.
The forces that initially draw 3HM molecules together into balls are weak, so Xu and her group expected 3HM to disintegrate quickly without further tuning. Instead, they found that 3HM held together incredibly well, and that the protein heads of 3HM create extra forces that bind 3HM together.
As a drug carrier, 3HM’s smaller size gives it an advantage over liposomes. In a recent study on rats, Xu and colleagues at UC San Francisco and UC Davis demonstrated that 3HM outperforms liposomes in reaching glioblastoma tumors. Why is 3HM better than other nanocarriers at crossing the blood-brain barrier? “We still don’t understand the mechanism completely,” says JooChuan Ang, a graduate student in Xu’s group. “Size is definitely a factor, but there could be other factors that contribute to the [movement] of 3HM across the blood-brain barrier.”
One thing is clear, however: 3HM boosts the potential of treating glioblastoma with nanocarriers. Just as the devastating glioblastoma started with a small change, its demise starts with tiny particles.
Dharshi Devendran is a postdoctoral researcher at Lawrence Berkeley National Laboratory.
This article is part of the Spring 2016 issue.



