
Natural selection is comparable to sorting through piles of clothes and picking out items to either put back in the closet or give away. The most practical or pleasing articles may go into the “To Keep” pile, as the unused ones are cast aside. If a biological trait is shared by a multitude of organisms—spanning species and evolutionary history—there is a good chance it is important for survival. And just as some fashion choices are baffling, some traits conserved by evolution don’t have obvious functions. The result is a hodgepodge of clothing, or segments of DNA containing instructions for various traits, that remain in the closet of genes based on nature’s discretion.
In the closet, one may find sets of genetic material that preserve methods of cell-to-cell communication, coding for proteins known as receptors. Like miniscule mailboxes, these receptors are situated on cell surfaces, where they are prepared to receive and convey information via chemical signals. Different types of receptors are stationed everywhere from the surface of a cell to its genetic-material-filled heart, the nucleus, where they provoke diverse cellular responses.
The average human body has about 37.2 trillion cells, so it would not be feasible for each of these entities to live blissfully unaware of their neighboring cells or their environment. The microscopic world of the cell, like human society, requires good communication in order to transmit information and respond adequately to situations that arise. Thus, cells must have the ability to meticulously manage the information they receive from both the environment outside as well as that inside. Cell receptors called G-protein coupled receptors, or GPCRs, are a part of this process, acting as a bridge between the exterior of the cell and its interior. Although GPCRs are one of the largest and most important classes of cell receptors, scientists have discovered the majority of what is known about them within the last 40 years.
Given the importance of these receptors, UC Berkeley scientists are part of the ongoing pursuit to better understand them. GPCRs are conserved across many species, so research in this field features diverse model organisms and technical approaches. From how the shapes of these receptors change in the brain, to their roles in the sex lives of yeast, to how zebrafish detect scents, there is not just one way to look at a GPCR. They play a critical role in human health, with their dysfunction implicated in disorders from heart disease to Alzheimer’s, and are a common drug target for pharmaceuticals. As a result, research into GPCR structure and activity not only provides insight into this ancient anatomical feature, but has ramifications for drug development as well. As imaging technology and molecular techniques advance, researchers continue to unravel the nature of GPCRs as receptors with archaic origins and modern day significance.
Receptors: fact or fiction?
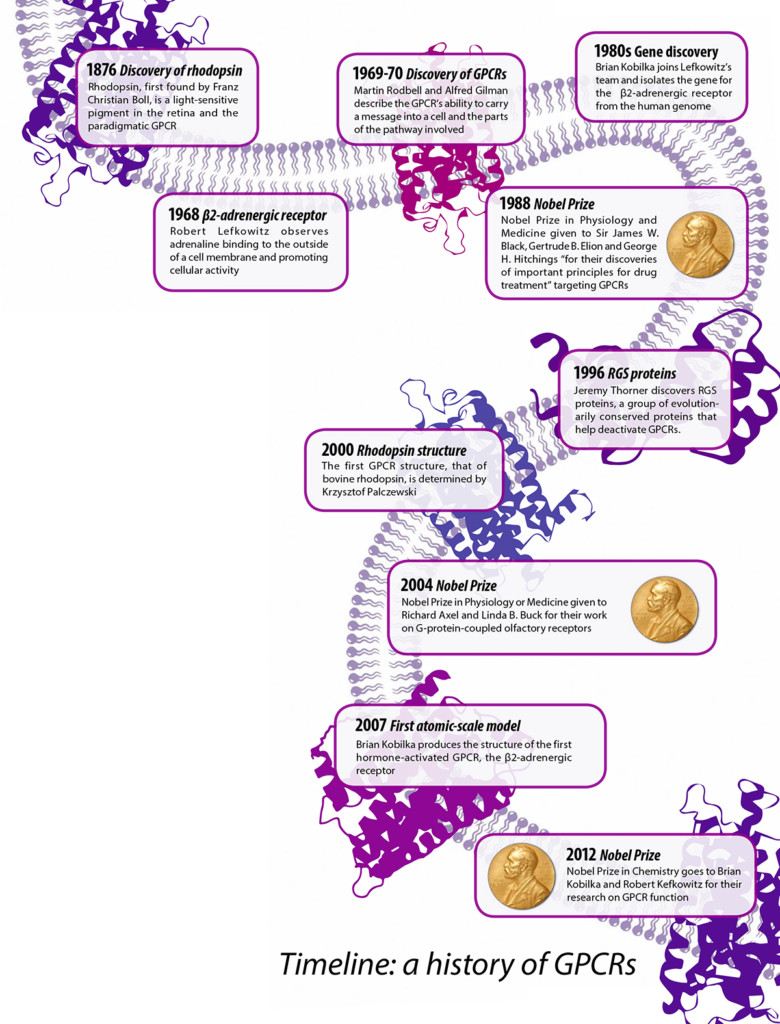
The anatomies and mechanisms of cell receptors were shrouded in mystery for over a century. Chemicals could effect change in cells, but how? Scientists found the idea of them curious, a reasonable explanation for observed biological phenomena, yet remained skeptical of a concrete “receptive substance” that could receive and convey signals. In 1968, Duke University researcher Robert Lefkowitz set out to identify the figurative mailboxes for various hormones. Using a radioactive version of iodine, he tracked these hormones as they homed in on their cellular targets. He found that adrenaline, a hormone often associated with the fight or flight response, did not enter the cell directly to deliver its message, but instead stuck to something on the surface of the cell. He called this “something” an adrenergic receptor. “There was no such thing as a G-protein in those days, and they weren’t called G-protein coupled receptors, of course,” Lefkowitz said. “Most people didn’t know they existed.” Early in his career, Lefkowitz pursued these receptors despite the general consensus that they were unidentifiable. Why? “We thought it was an idea that was worth exploring,” he said.
Lefkowitz shared a Nobel Prize for this work with Stanford University’s Brian Kobilka, who started working under Lefkowitz as a young postdoc and later discovered the gene that controls the production of this receptor. The two contributed crucial information on how GPCRs function, even before these proteins were identified as GPCRs.
However, doubt remained even after the chemical activities of these GPCRs were officially documented. In 1973, years after he himself had written an article about adrenaline receptors, prominent pharmacologist Raymond Alquist described them as more of an “abstract concept,” conceived to describe bodily responses to chemicals. He also brushed off efforts to describe their structure. At his Nobel lecture, Lefkowitz recalled Alquist’s comment. It is now known that these receptors “regulate all known physiological processes in humans,” Lefkowitz said with gravitas. GPCRs manage essentially all of our senses and organ functions, and are the targets of half of all prescription drugs.
 TIMELINE DESIGN: JUSTINE CHIA; NOBEL MEDAL: ®© THE NOBEL FOUNDATION; RHODOPSIN AND Β2-ADRENERGIC RECEPTOR ADAPTED FROM S. JÄHNICHEN
TIMELINE DESIGN: JUSTINE CHIA; NOBEL MEDAL: ®© THE NOBEL FOUNDATION; RHODOPSIN AND Β2-ADRENERGIC RECEPTOR ADAPTED FROM S. JÄHNICHEN
A trio to transmit messages
Studying the details of GPCRs is difficult: they are extremely small, and viewing one is not as easy as looking into a microscope. According to Lefkowitz, isolating them is “extraordinarily difficult work.” To separate GPCRs from other cellular components, they must be removed from the cell membrane and purified. GPCRs exist as a molecular complex, a group of molecules that tightly associate with one another. They loop through the cell membrane seven times, giving them the alternate name seven transmembrane receptors.
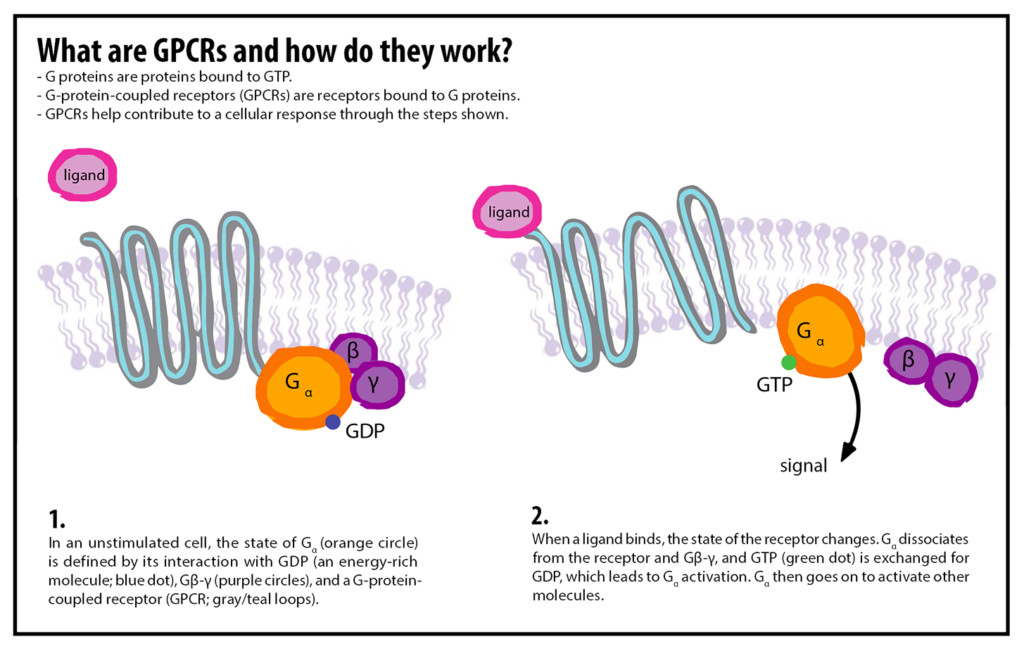
In 1971, researchers identified three components required to transmit a message into a cell, and thus, the three parts of a GPCR: a discriminator (receptor), a transducer, and an amplifier. First, a chemical signal outside the cell, known as a ligand, fits into the binding pocket of a GPCR in a lock-and-key fashion, initiating a chain reaction. For example, adrenaline is the ligand that binds to the GPCRs studied by Lefkowitz.
Second, once a ligand attaches, the GPCR changes shape and activates the G-protein coupled to it. A guanosine triphosphate (GTP) molecule, acting like a charged battery, attaches to the G-protein. Third, the transducer, here the G-protein, propagates the signal like a game of telephone. It breaks apart into smaller units that move around within the cell membrane, activating systems that amplify this signal. These amplifiers produce other signals that travel throughout the cell, turning different processes on or off. If any of these three components are missing, the incoming messages, like adrenaline, will no longer have an effect on the cell.
 DESIGN: JUSTINE CHIA; ADAPTED FROM 10.1038/NATURE0130
DESIGN: JUSTINE CHIA; ADAPTED FROM 10.1038/NATURE0130
The chemicals produced within the cell by these amplifiers made studying GPCRs possible before the receptors were even defined. “There was an easily measurable, cellular, functional output that I could look at,” Lefkowitz said. At UC Berkeley, researchers have also found creative ways to study these receptors, and there still remains much to discover.
Quick switches
Since GPCRs are usually embedded in the cellular membrane, their structures can be obscure, but new technologies are allowing researchers to study how they work. Reza Vafabakhsh and Joshua Levitz, postdocs at UC Berkeley from the laboratory of neurobiology professor Ehud Isacoff, have studied how GPCRs become activated, a patchy area of knowledge.
Picture a dance studio where everyone is working separately on the same choreography. Each dancer sways at a different tempo, without synchrony. A full view of the studio action will give the impression of disorder, but if you focus on an individual dancer, his or her movements become definable. From this perspective, you can now observe the same sequence of moves being performed by each person.
This is the basis of a technique known as single-molecule pull-down, which Vafabakhsh and Levitz used to prepare samples of individual metabotropic glutamate receptors (mGluRs), a class of GPCRs found in the human nervous system. Single-molecule pull-down involves selectively capturing a single molecular complex—in this case, a GPCR—from a cell extract, and using a microscope to image it for analysis. Vafabakhsh and Levitz attached fluorescent molecules to different parts of the GPCR to observe how the shape of the receptor changes upon receipt of a molecular signal.
“If you have a protein that goes between multiple states—one, two, and for a brief moment of time, three—if you average everything, you’re not going to see state three, because it’s very brief,” Vafabakhsh says. By creating this window and analyzing individual activity rather than looking at a cluster of GPCRs, the researchers identified three distinct receptor states: resting, activated, and a short-lived intermediate.
This experiment was not easy. “Inherently these proteins are big. They’re wobbly—they have lots of moving parts. You can’t freeze them. You have to use a lot of tricks,” Vafabakhsh says. The experiment took a couple of years and was the first to use single-molecule pull-down on a GPCR. “With techniques like this you can now see the in-between parts too,” he said.
These mGluRs naturally respond to the binding of the ligand glutamate, a very important molecule in the nervous system. This particular class of GPCRs is associated with a range of neurological disorders, from depression to Alzheimer’s, and serves as a drug target. A deeper understanding of GPCR structure could play into the discovery of more targeted drugs—perhaps one that can take advantage of that short-lived intermediate. Not only do the findings of Vafabakhsh and Levitz have implications for drug discovery, they have also introduced new methods that can be used to study other GPCRs.
Management of cell-cell chatter
Ever look at yeast and think, “We’re not so different, you and I”? If not, now is the time. Eukaryotes—the group of organisms that includes animals, plants, and fungi—all have GPCRs, whether they are single-celled organisms or multicellular animals. Scientists know that the human genome codes for over 800 of these receptors, yet around 150 of them still have unknown functions. Although not all eukaryotes share the same types of GPCRs, some of these receptors have similar functions in different organisms.
In 1974, biochemist Jeremy Thorner began his career at UC Berkeley as an assistant professor. Enticed by the mystery of cell-cell communication, he sought a simple organism on which to base his research, particularly one that resembled the popular model bacterium Escherichia coli (E. coli). Leaning away from furry laboratory specimens, he selected Saccharomyces cerevisiae—also known as budding yeast, the same fungus that raises bread and ferments grain to form beer. The genetics of yeast are simple to manipulate, Thorner says, and plus, they don’t bleed or scream. “Everything that’s alive on this planet is connected to everything else by the process of evolution, so it makes it worth using model organisms,” Thorner says.
Yeast can exist as three different mating types, two of which can fuse in a way similar to the union of a sperm and an egg. In order to fuse—and therefore reproduce—the two mating types must locate each other. How do they know when to mate? They use chemical signals to say: I’m here and I’m feeling frisky.
These yeast cells secrete mating pheromones, which act as ligands that bind to distinct GPCRs. The fact that GPCRs exist in yeast suggests an early evolutionary origin. Like humans, yeast cells have a nucleus and specialized cellular structures. Although they are unicellular, knowledge of GPCR functions in yeast may be applicable to humans, despite having evolved separately for about a billion years.
Thorner uses yeast to investigate the nitty-gritty of GPCR signaling. Once a signal is turned on, its strength and duration must be controlled. Cells also need to be able to turn it off at the appropriate time. To do so, the cell clears out old signals and resets parts of the GPCR, much like re-gating a waterway after opening a dam.
This resetting needs to happen in both yeast and human cells so that new signals can be properly received. Imagine, for example, that you are walking out of a movie theater after two hours in the dark. The door opens, light pours in, and you are blinded. You squint for a few minutes as you walk to your car, eyes aching. Slowly, your eyes readjust. “The intensity of the sun hasn’t changed in that minute or two. The ability of the receptors to send a signal has been down-regulated, so they don’t send as potent a neuronal signal to your brain,” Thorner explains. Hence, the receptors on the cells in your eyes—which are also GPCRs—are no longer as sensitive as they were when you were sitting in the theater.
In the 1980s, Lefkowitz identified multiple proteins involved in these processes of desensitization and adaptation. One of these is the G-protein coupled receptor kinase (GRK). In an effort to dampen GPCR signals once they are activated, GRKs add specific molecules to a receptor to stop its activity. The rest of the route to desensitization can vary. A type of protein called an arrestin may link to this receptor and (true to its name) prevent it from attaching to the G-protein for the final hurrah, temporarily shutting off the GPCR.
A decade later, a study done by Thorner identified another piece of this puzzle: a group of evolutionarily conserved proteins in yeast known as regulators of G protein signaling (RGS). An RGS slows down GPCR activity by draining the GPCR’s figurative battery, GTP. Prolonged stimulation of a yeast cell by mating pheromone can hurt the cell, but yeast has an RGS protein that can reset the GPCR activated by mating pheromone. This desensitization process prevents the yeast from being frozen in the reproductive stage of its life cycle, allowing it to resume its normal growth and division.
If an organism is exposed to a signal, it needs to initiate an appropriate response. RGS proteins and other molecules help standardize this response even if it can be triggered by varied conditions. Lacking this ability to adapt may lead to overstimulation, as is the case when the overreaction of the human immune system leads to inflammation and allergies. Efficient regulatory systems help organisms function in less than ideal environments, or simply, to adapt.
The sensory world of GPCRs
UC Berkeley professor of neuroscience John Ngai says that GPCRs enable organisms to “navigate a sensory world.” Although most research on GPCRs is fairly recent, scientists discovered the light-sensitive protein in our eye, rhodopsin, as early as the 1870s. With its gene sequenced in the 1980s and structure partially determined in the 1990s, rhodopsin became biology’s model GPCR about 30 years ago, and remains the best-understood member of this class of receptors. Much of your ability to detect light and hence your eyesight can be attributed to this membrane receptor. As the first GPCR structure available, cow rhodopsin has acted as a model for GPCRs in general, and for therapeutic drugs treating GPCR-related disorders in humans.
Rhodopsin is found in your retina—a light-sensitive layer of tissue in your eye— and responds to low-light conditions. When light enters your eye, rhodopsin undergoes a structural change and activates the G-protein, which transmits a signal to the rest of the retina and, eventually, the brain.
GPCRs also play a role in your other senses, including your sense of smell. Humans have around 350 olfactory receptors that can (theoretically) detect over 1 trillion distinct odors. To study the role of GPCRs in the olfactory system, the Ngai lab uses a striped, slippery creature called a zebrafish, which can smell and is better suited for certain laboratory tests than mammals. For one, it is easier to work with scents in a liquid environment than air since the olfactory system requires a fluid to detect scents (in humans, that role goes to mucus). Zebrafish embryos develop on the outside of a spawning fish, so they are easily removed and raised in a Petri dish. Lastly, the fish are transparent, so visualizing their nervous systems and even neural circuits under a microscope is uniquely convenient. Zebrafish have about 150 genes for olfactory receptors, a more manageable number to study than the 350 in humans or the 1,400 in rodents.
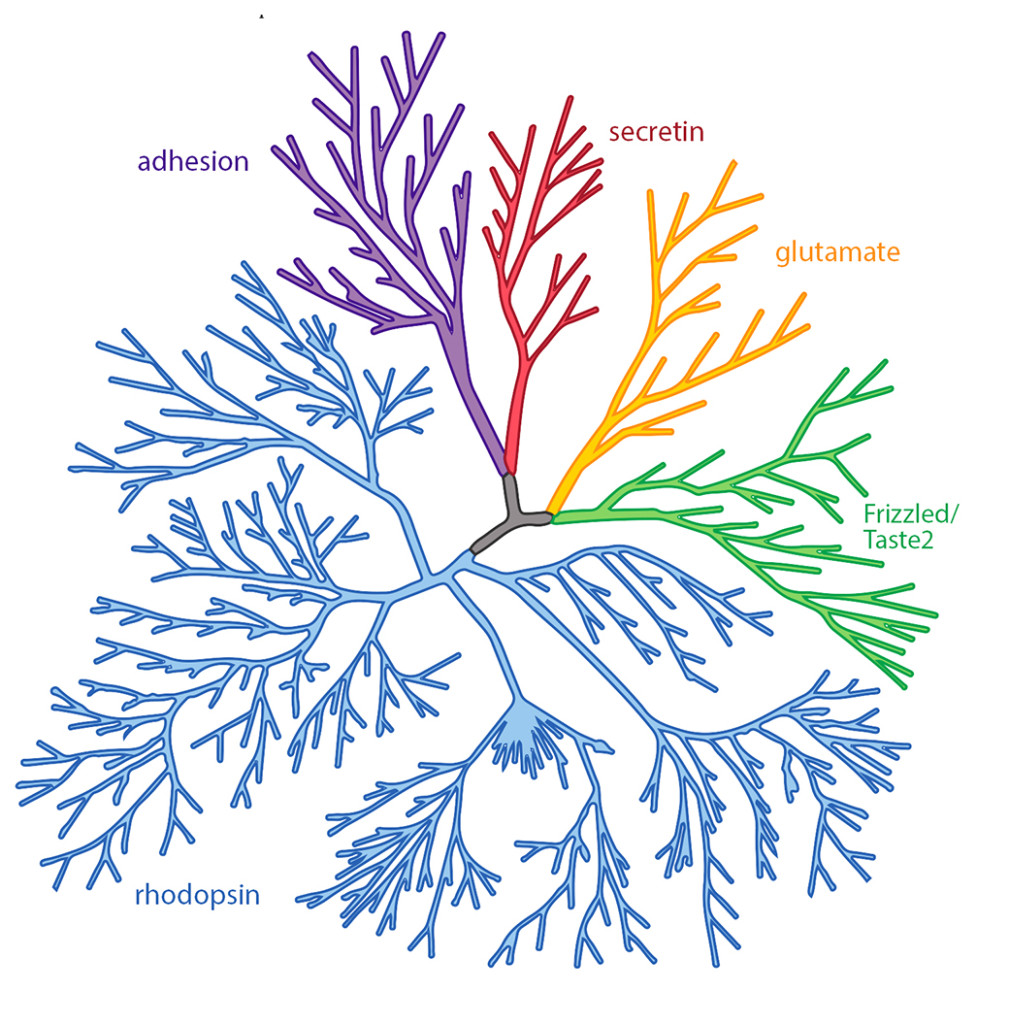 *Phylogenetic tree representation of the human GPCR superfamily. Scientists divide GPCRs into 5 classes based on their sequence and structural similarities. These classes are secretin (red), glutamate (orange), Frizzled/Taste2 (green), rhodopsin (blue), and adhesion (purple).*
*Phylogenetic tree representation of the human GPCR superfamily. Scientists divide GPCRs into 5 classes based on their sequence and structural similarities. These classes are secretin (red), glutamate (orange), Frizzled/Taste2 (green), rhodopsin (blue), and adhesion (purple).*
The particular olfactory receptor on a neuron determines which scents it can detect, Ngai explains, and each neuron only produces one receptor type. For instance, if a neuron has the option of three different types of receptors (X, Y, and Z), once it produces X, it must block the genetic expression of Y and Z to prevent their production. “In terms of gene regulation, this is a fascinating problem. The [neuron] not only needs to choose which receptor to express, it needs to forsake all others,” he says.
Olfactory neurons are thus highly competent at selecting only one type of GPCR. A 2014 study carried out by Ngai and his colleagues used zebrafish to identify how the neuron makes this selection: the chosen olfactory G-protein itself can prevent the addition of any more receptors. Once the cell starts using a specific receptor, it essentially bans all others by blocking the genes that code for them.
Today, Ngai’s lab continues to study zebrafish. One current project is looking at an automatic fear response to an alarm pheromone, which gets released by injured fish to warn of a nearby predator. “Eventually we’d like to identify the receptors that receive and mediate the alarm pheromone response,” Ngai said. “The receptor may—or may not—wind up being a GPCR. Time will tell.”
Drugged
Time will also tell whether additional GPCR protein structures are on the horizon. As common drug targets, the molecular details of these receptor complexes provide valuable information. “The gold standard for drug discovery is structure—if you have the structure, then you know where the compound of interest will bind,” Vafabakhsh said. “But also, if you have the structure, you can do this drug discovery in your computer.”
Drugs were targeting G-proteins before the receptors were even discovered. The first beta-blocker, propranolol, was developed in 1964 to treat blood pressure. It turns out the drug slows the heart by blocking the attachment of adrenaline to adrenergic receptors. Even though our knowledge of these receptors has improved, current methods of drug discovery can be disorderly. GPCRs may be exposed to a library of compounds, sometimes at random, to see if they respond.
Without a physical structure at hand, drug discovery can be like finding a key for an unknown lock—a process of trial and error. The goal might vary based on the type of drug one is seeking: “you can always make a key that fits into multiple locks, and you can make a key that only fits into one,” Vafabakhash says. If you’re only trying to target a particular GPCR, knowing what distinguishes that particular receptor from a different type of GPCR could make all the difference in designing a better, more specific drug. For instance, the schizophrenia drug olanzapine (Zyprexa) may target at least a dozen GPCRs. Some of olanzapine’s broad effects may help the sufferer, while others may cause unwelcome side effects like weight gain.
Since GPCRs play so many roles in the body, unwanted changes in these receptors caused by pathogens, as well as genetic conditions, can lead to disease. Vibrio cholerae is a pathogen that causes cholera, and is spread by contaminated food or water. This bacterium provokes an acute intestinal infection via a toxin that locks an intestinal cell’s G-protein in its active form, stimulating a heavy flow of water from the blood into the intestines and causing diarrhea.
The number of G-proteins present— having too many or too few—can also lead to disease. Certain symptoms of alcoholism and diabetes are attributed to improper G-protein number, and in some types of cancer, tumors produce extra GPCRs. Modifications of adrenergic receptors, which may lead to abnormal GPCR proteins, are linked to common diseases like asthma and heart failure.
But with gaps in our knowledge of GPCRs, certain attempts at drug discovery are shots in the dark. When studies highlight important parts of a GPCR, these details may be summed up and fit into entirely hypothetical GPCR structures, which may not be perfectly accurate. But future studies might identify GPCR “druggable sites,” with important implications for health and medicine. New imaging technology and advancements in methods like crystallography, which allows researchers to determine the precise shapes of molecules, may lead to a new era of drug discovery.
Over his 40-year career as both a physician and researcher, Lefkowitz has seen GPCRs go from nonexistence to a major breakthrough in pharmacology and physiology. “I think at a translational or practical level, the game has always been to develop more and more specifically active drugs; that is, drugs that will have more specific effects and fewer side effects,” he says. “The key to that is understanding as much as we can about the basic structure and workings of these receptors.”
Indeed, GPCR structure is no longer just a curiosity. Thorner has spent his entire career studying these seven transmembrane receptors, and still hopes to see more GPCR structures solved. “This mode of communication—releasing a soluble, diffusible substance from one cell that acts on another cell—is a very evolutionarily ancient process,” Thorner said. Nature has kept them in the gene pool (or gene closet) for a reason.
Cover image credit: Yekaterina Kadyshevskaya, Bridge Institute, University of Southern California
This article is part of the Spring 2016 issue.



