If you have ever made the roughly 2.5-mile trip from the UC Berkeley campus down to the Berkeley Marina, you probably did not notice the unassuming olive-green building sandwiched between the train tracks and the water. That’s because it is not trying to look pretty or to attract passing customers, but is instead meant to provide the basic facilities for young start-ups trying to get their feet off the ground. Inside one of these spaces you can find Alexander Teran, a 2013 graduate of the Department of Chemical Engineering PhD program at UC Berkeley, and the chief battery engineer and cofounder of a start-up called Blue Current.
Blue Current wants to make electric vehicles competitive with conventional petroleum-powered modes of transport. For electric vehicles to succeed, not only must they be cheaper, but they must also be safer. To Blue Current, the heart of any electric vehicle—the battery—is a big liability on both counts. Lithium-ion batteries (LIBs), the standard rechargeable batteries used in everything from cell phones to electric vehicles, include a highly volatile and highly flammable organic solvent. This is fine if the battery works as it should, but battery failure—whether due to manufacturing defects or external abuse—is unavoidable, and can result in an uncontrollable rise in temperature that vaporizes the solvent and pressurizes the battery. Scaled down to the size of a cell phone or laptop, the consequences are minor, but scaled up to a car or an airplane and the consequences can be catastrophic. Companies such as Tesla and Boeing have had to design around this critical flaw, increasing the level of complexity and price of the resulting product, but the safety risks still linger. Blue Current wants to change this.
Such lofty goals, however, often have humble beginnings. Back in September of 2014, when the company first moved in, you would have found Teran working alone in the barren unit. Instead of research, the early days were filled with administrative duties such as setting up the wireless network, sorting through applications of potential employees, and completing paperwork for hazardous waste disposal. This can be expected when you are the first and only employee. The only items filling the unit were rows of empty laboratory benches and souvenirs left behind from the biotech start-up that had previously occupied the space but failed to make it off the ground: fermentation units, gas regulators, and ill-fitting lab coats, among others. Although mostly useless to Blue Current, they served as a silent reminder of what happens to most start-ups.
The odds do not seem to faze Teran, who has already experienced the fall of a start-up since graduating from UC Berkeley. Out of graduate school he took a job as a process technologist for Leyden Energy, a mature battery start-up in Fremont with about 50 employees and seven years of experience. Like Blue Current, the focus at Leyden Energy was on LIBs.
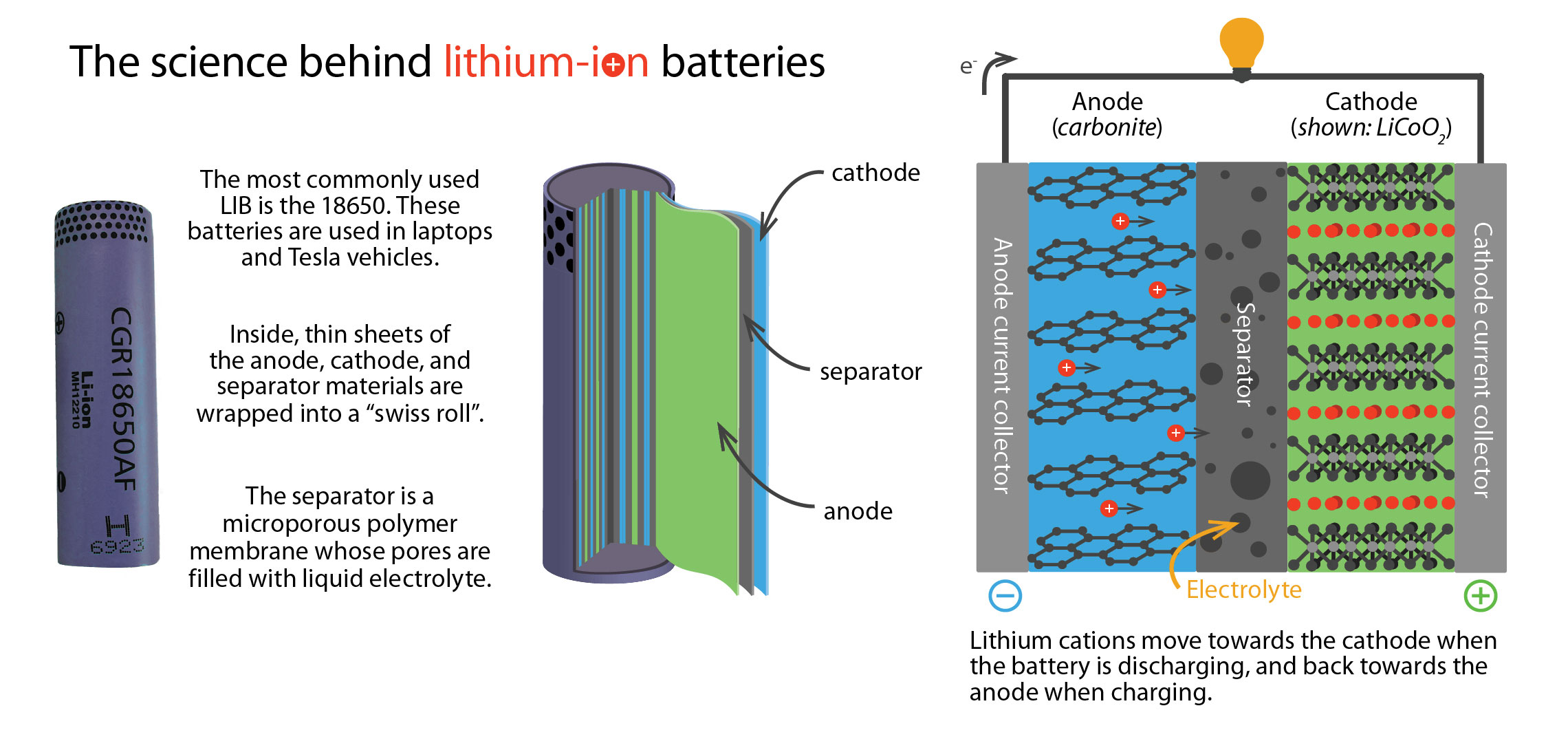
Typical LIBs are composed of a liquid solvent, called the electrolyte, that is sandwiched between positive and negative electrical conductors, called electrodes. This electrolyte is the flammable component Blue Current wants to replace. During discharge, or use, lithium ions (Li+) flow through the electrolyte from the negative to the positive electrode, while negatively charged electrons make the same journey but through an external circuit. This flow of electrons is the electrical current that powers your device. The reverse process occurs when charging the battery. Connecting all these components to produce a working battery is an intricate process, with seemingly minor variations greatly affecting the overall performance. At Leyden Energy, Teran was tasked with developing an understanding of the key manufacturing variables needed to improve the fabrication process.
 Design: Holly Williams; battery: Lead Holder; lightbulb: simpleicon.com. Click to enlarge.
Design: Holly Williams; battery: Lead Holder; lightbulb: simpleicon.com. Click to enlarge.
No more than six months after he started at Leyden, however, the company had to close its doors. By then, Leyden Energy had been working for seven years on improving the current state of LIBs via two distinct projects: improving the thermal stability and power performance of LIBs for automotive applications, and developing a negative electrode that boosts the energy density of LIBs destined for consumer electronics. Although useful, research alone does not bring in any money.
“Part of the problem with the battery industry, or hardware-based start-ups in general, is that you spend a lot of time and money developing a technology,” explains Teran. “Even if you have a technology that’s better than anything else that’s on the market, to actually manufacture it is not a guarantee by any stretch.” Leyden Energy had gotten their products to work at the pilot scale, but without any in-house large-scale manufacturing capabilities, they needed to partner with a larger company. There were several interested parties, but with each battery sample that Leyden Energy sent out, only more questions and requests for further testing came back. The process dragged on. “It’s easy for these big companies to wait until you run out of money, and then they can just get whatever you were trying to sell for less money,” says Teran.
Blue Current, he thinks, will be able to avoid some of the difficulties and pitfalls faced by Leyden Energy. For instance, instead of splitting the company’s energy between two goals, its focus lies solely on improving the liquid electrolyte. Replacing the electrolyte with a nonflammable alternative has been a dream for years, but has always resulted in poor performance. Luckily, new research from UC Berkeley, in collaboration with the University of North Carolina at Chapel Hill (UNC), has found a promising alternative: perfluoropolyethers (PFPEs).
From lubricants to batteries
Professor Joe DeSimone of the chemistry department at UNC and Professor Nitash Balsara of the chemical engineering department at UC Berkeley were fans of each other’s work, and had been looking for excuses to collaborate for years. When DeSimone visited UC Berkeley to give a talk, the opportunity presented itself. DeSimone discussed his group’s work on PFPEs, common lubricants in industry, and their potential use on the bottom of naval ships and docks to prevent marine life from collecting. He had an additional application in mind, however.
PFPEs mixed favorably with polyethylene oxide (PEO), a nonflammable polymer electrolyte studied by the Balsara group as a safer alternative for current LIBs. With a high crystallization temperature, however, PEO-based electrolytes are solid at typical operating conditions, thus limiting their applicability. Blending PEO and PFPEs together decreased the crystallization temperature, but hindered performance. “The electrolytes were lousy,” recalls Balsara. “They are not very interesting when you make blends.” He decided to keep pursuing PFPE-based electrolytes, however, because they had a very unique and alluring property.
“You could hold a flame to it and it wouldn’t propagate the flame,” says Jacob Thelen, a fourth-year chemical engineering graduate student in the Balsara lab who collaborated with Dominica Wong from the DeSimone group on the project. In fact, PFPEs would not even vaporize below 200°C. This is in stark contrast to conventional LIB electrolytes, which produce flammable vapors at temperatures as low as 24°C.
 Alex Teran and colleague work in glove boxes. Credit: Keith Cheveralls. Click to enlarge.
Alex Teran and colleague work in glove boxes. Credit: Keith Cheveralls. Click to enlarge.
The key to these nonflammable properties lies in the high fluorine content of the polymer backbone, but it comes at a heavy price. Alone, commercial PFPEs are useless as an electrolyte because the fluorine atoms decrease the polymer’s ability to dissolve positively charged lithium. Li+ is the work-horse in LIBs, shuttling between the electrodes during charge and discharge. PFPEs could not even dissolve enough Li+ to make it worth testing as an electrolyte. Thelen and Wong decided to chemically alter the PFPE structure by incorporating a component that interacts favorably with Li+: dimethyl carbonate (DMC). Using this approach, they were able to more than double the concentration of dissolved Li salts, but would the Li+ in the system be able to flow?
“There are tons and tons of polymers that dissolve salts but have zero conductivity—they’re just stuck,” explains Balsara. Conductivity, or flow of ions under an electric field, is an important criterion that helps determine the viability of a new material as an electrolyte. It turns out that the conductivity values in the PFPE system were fair, albeit two orders of magnitude lower than conventional electrolytes. Conductivity, however, is only part of the story; lithium salts are composed of both positively charged Li+ and negatively charged counter anions. Their respective contributions, however, are indistinguishable using standard conductivity measurements.
“In a battery you only really care about Li+ moving around because the [counter ion] is just a spectator ion,” explains Thelen. To fully characterize their electrolyte system, Thelen and Wong needed to know the fraction of the current produced by Li+, known as the transference number (t+). Working with Didier Devaux, a postdoc in the Balsara lab, they found this number to be near 1, indicating that the spectator anion was largely stationary while Li+ flow was responsible for most of the current. This was a highly unexpected result, as conventional electrolytes have t+ values below 0.5. According to theoretical calculations, the high t+ of the PFPE system is very promising, as it may offset a relatively low conductivity and increase battery life. “A battery with a high transference number [can] get better performance, even if the conductivity is lower,” says Thelen. “It always depends on how you want to design your battery, and how you want to optimize it for a given application.”
Using their electrolyte, Wong and Thelen constructed laboratory-scale batteries that showed promise for the electrolyte’s compatibility with standard battery components. Being able to replace the flammable electrolyte currently used without having to redesign the other pieces of the battery is advantageous.
The mystery remains, however, why these polymers have such a remarkably high t+. Thelen and his colleagues hypothesize that the fluorinated backbone, which was unable to dissolve Li+, may be attracting the spectator counter ion of the lithium salt, anchoring it in place while Li+ flows by. It is also possible that the fluorinated backbone shifts electron density in the polymer such that its attraction to Li+ is weakened, increasing its mobility.
Although unraveling these unknown transport characteristics will be the subject of future study for DeSimone and Balsara, the two recognized that such academic pursuits were not required to start commercially developing a better, safer battery. The electrolytes used in the study, however, were not ready for consumer use. Thelen had several ideas for improving their performance. To increase the conductivity they could switch the lithium salt to one that is more soluble, or alter the polymer backbone such that it dissolves more lithium salt. Then, of course, they could start playing around with what he calls “black magic”—various additives that result in orders of magnitude improvement. This is, however, where the slow pace of theory-driven academic research and the rapid fire pay-to-play of a commercial lab go their separate ways.
“I haven’t really gone more in depth since all of the research is more what you would do in a company,” explains Thelen. “You just need to try things and go—not a good PhD project.” Not only would it be poor fodder for a thesis, but such research also requires sophisticated equipment in order to be done efficiently and systematically. This type of work is beyond the budget and scope of a typical academic lab.
A start-up is born
 Design: Holly Williams; data: UC Office of the President; map: Kryston. Click to enlarge.
Design: Holly Williams; data: UC Office of the President; map: Kryston. Click to enlarge.
“Things can happen a lot faster in start-ups than they happen in academic labs,” reasons Balsara. “There comes a point in your R&D when you think that this thing is important. In some sense you want the answer quickly.” Balsara is not alone in this type of reasoning. Over 162 UC Berkeley licensed startups have been created since the mid 1990s. Carol Mimura, the Assistant Vice Chancellor for Intellectual Property and Industry Research Alliance (IPIRA) at UC Berkeley, says that it is not uncommon for professors to straddle the fence between research and industry.
IPIRA handles all invention disclosures stemming from UC Berkeley. Their job is to determine if the invention is patentable—meaning new, useful, and unobvious—and whether any company would actually want to license such intellectual property (IP). Sometimes, however, inventions are before their time, or can only be commercialized with the passionate drive of the inventor who has the proper expertise.
“In that case that kind of IP right is appropriate for licensing to a start-up,” explains Mimura. “Essentially something that existing companies would feel are too risky—too uncertain—to commercialize.” Big companies can’t invest the time and money required to bring every nascent technology to market, but academia is often incapable of bridging the gap. This is where start-ups such as Blue Current come in.
Mimura cautions, however, that university start-ups face many challenges. Professors making the transition from academia are often unfamiliar with entrepreneurship, or have become too accustomed to the competitive, every-man-for-himself culture at top-level research institutions. “It’s difficult for many career academics to transition directly into start-ups, work well in teams, and be flexible with pivot-changing directions,” she says. “They tend to hold on too strongly to the original idea or are not open to what their board is telling them is important because they are used to being top dog in the lab.” For professors without tenure, a start-up can also be a major time sink that may not hold any clout in the eyes of their colleagues who grant tenure.
Raising money is another major hurdle. Angel investors who invest in often risky start-ups in return for ownership not only help solve this issue, but also come with experience and contacts that can help make their investments fruitful. Mimura calls it smart money. “Because of the early stage of the companies that they invest in, they really do babysit those investments and help manage the company,” explains Mimura.
Luckily, DeSimone had connections to angel investors who were interested in Blue Current, and both he and Balsara had previous experience founding start-ups stemming from research in their group. With smart money in their pockets and experience under their belts, the two professors decided to add another company to their portfolio. After patenting their technology, publishing their work, and coming to a licensing agreement between all parties involved, Blue Current was born.
Now what Balsara needed was a battery engineer to get Blue Current off the ground. Luckily, an alumnus of his research group had just recently come back on the job market, and was willing to take on the risk. Teran got the job.
Unlike his counterparts in academia, Teran’s focus will not be on answering theory-driven questions regarding the mechanism of ion transport through the electrolyte, but instead on developing a working device. One of his biggest challenges will be integrating the electrolyte with commercially-relevant battery electrodes.
 Design: Holly Williams; data: Tesla Motors; Roadster: Fogcat5. Click to enlarge.
Design: Holly Williams; data: Tesla Motors; Roadster: Fogcat5. Click to enlarge.
“Once you incorporate these materials into a battery, they have to play nice with the electrodes,” explains Teran. In particular, it is necessary to form a stable solid electrolyte interface (SEI) between the electrolyte and the electrodes. The SEI prevents electrolyte decomposition, increasing the amount of current that the battery can handle and preventing failure. Sacrificial additives, which get consumed in the first charge to form the SEI layer, are a common solution, but the trick lies in figuring out which ones to use and how much. “There’s a lot of work to be done to understand how these PFPE materials, which are very different than conventional electrolytes, behave with regards to the SEI layer.”
Experimenting with different materials to improve conductivity is also on the to-do list, and buying industrial battery testers that can test 128 prototypes at once will help Blue Current determine the optimal parameters. This is a luxury that Thelen and his colleagues would not have had access to at the university level. Conversely, Blue Current does not have the luxury of time to worry about the underlying mechanism behind the high t+. For Balsara, who advises both his lab at UC Berkeley and Blue Current, these distinct interests make it easier to keep information learned by one party from the other. In fact, he’s contractually obligated to do so. He thinks of it as having a “wall in [his] brain” that separates the two.
Ingredients for success
Despite further experiments required to improve the PFPE system, Teran is optimistic that Blue Current will be able to overcome some of the challenges of other battery start-ups like Leyden Energy. One major benefit is their narrow focus on the electrolyte, which is compatible with current LIB chemistries and can be used as a drop-in replacement.
“There are other battery start-ups where their technology might be really interesting, but it involves completely reinventing how the batteries are manufactured,” explains Teran. “You have to basically build the battery from scratch.” Not only does the electrolyte’s compatibility allow Teran to test his batteries using standard, off-the-shelf materials and components, but it also makes the technology more attractive to battery manufacturers. Such companies would be able to incorporate Blue Current’s new electrolyte seamlessly. This is a key advantage, as Blue Current needs to gain attention from these established companies in order test their electrolytes on a larger scale and, ultimately, to succeed. Teran understands this well from his time at Leyden Energy. Convincing end users such as Tesla and Boeing of the technology’s value is also important since they can ultimately sway their battery suppliers to work with Blue Current. Such companies are, after all, the ones who would benefit most from non-flammable electrolytes.
“It’s an amazing statistic that only one in 10 million cellphone batteries fail,” says Balsara. “However, that kind of statistic—when extrapolated to a car—will give a failure rate of one in 10,000, which in my view makes it unacceptable.”
Cars or planes that catch fire are, of course, not going to sell very well, and the companies interested in larger battery-powered devices have gone to great lengths to engineer sophisticated control systems that prevent such catastrophes. According to Balsara, the 7,000 batteries in a Tesla each have their own temperature-measuring device, and the car is equipped with the equivalent of two refrigerators to cool down any battery that goes above temperature. “They spend a lot of money and time making a dangerous product safer, but if we can make these products inherently safe we can eliminate a lot of extra monitoring equipment and make them cheaper as well,” he says.
For businesses both big and small, cutting costs and being efficient is always a top priority. Blue Current has this in mind when choosing to keep operations small, reduce overhead, and narrow their focus on the PFPE electrolyte. Teran believes this strategy will be critical for their success.
For Balsara, the positive experience for his students, both new and old, already makes Blue Current a success. Wong and Thelen have learned how to characterize a novel electrolyte and how to construct batteries for testing, while Teran has learned that getting a start-up off the ground requires him to take on the role of human resources representative, information technology associate, and even custodian. The two separate approaches to the shared goal of producing better batteries will hopefully benefit everyone with a safer and greener ride.
 Alex Teran shows off Blue Current's progress after 5 months.
Alex Teran shows off Blue Current's progress after 5 months.
Onwards and upwards
Five months in and the unassuming olive-green building housing Blue Current looks as dull as ever, but inside the level of activity has increased significantly. Two employees now accompany Teran, with a third joining soon, and the company now has two start-up roommates that rent their extra space to defray overhead costs. Empty lab benches and rooms have been filled with battery testers, viscometers, two glove box units named Marge and Homer, and even a coffee break area with mugs sporting the Blue Current logo. More important than their swag, however, is their progress towards improving the PFPE electrolyte.
“[Initially] we basically had a paper that was published with very preliminary results, and it wasn’t clear how well that would translate to real batteries,” recalls Teran. “In the last [couple of] months we’ve made a lot of progress improving the performance of the base material.” This means improving the conductivity while maintaining the non-flammability that makes these electrolytes so interesting. Details of how they are doing this are, of course, kept secret, but Teran is confident that they have been getting good results. Blue Current is in the process of filing additional patents to protect those discoveries.
In addition to improving the electrolyte material, Blue Current has also refined the process of making batteries using their electrolytes. When dealing with new electrolyte materials, the first step is to make half cells that isolate one electrode at a time to make things easier to study. Blue Current has moved past this step, and is now constructing full battery cells. Despite the added level of complexity, Teran is excited about the development.
“This is a big transition for us as a company,” says Teran. “A few more months of work and I think we’ll have some really impressive cells to start shopping around and talking to people about.” For Teran, this is a thrilling but unfamiliar next step. The connections and influence of their angel investors, as well as DeSimone and Balsara, will be crucial. Taking off the lab coat and dealing with investors may not be familiar for a PhD chemical engineer, but this is just one more skill Teran will have to master to make Blue Current a success story.
This article is part of the Spring 2015 issue.



