
In 1888, Albert Baur was hard at work trying to make things explode. It had been 30 years since trinitrotoluene (TNT) was created, and scientists wanted a chemical that packed a bigger bang. What Baur created was decidedly less explosive than TNT, but it had one advantage—a distinct smell. His chemical creation had a warm, sensuous fragrance that he christened “Baur musk.” It would go on to be the base of many popular perfumes and launch the multibillion-dollar world of synthetic smells.
Because of advances in organic chemistry in the second half of the 19th century, several other synthetic fragrances were born alongside Baur musk, bringing about a turning point in perfume making. Synthetic fragrances were cheaper to produce than natural fragrances and could be mixed with harsh chemicals like detergents without breaking down, so fragrance companies started using synthetic methods to make perfumes.
Over time, this approach has blossomed into a gigantic, highly competitive, and highly technical industry. Fragrance companies and enthusiasts constantly pursue new technology for producing even better fragrances, which often takes them into cutting-edge scientific fields such as genetic engineering. Research at the Joint BioEnergy Institute and Lawrence Berkeley Laboratory has demonstrated the potential of using genetic engineering to make fragrances, and fragrance companies have taken notice. With scientists and fragrance companies working together, perfume making has become a fascinating interplay between science, business, chance, and creativity.
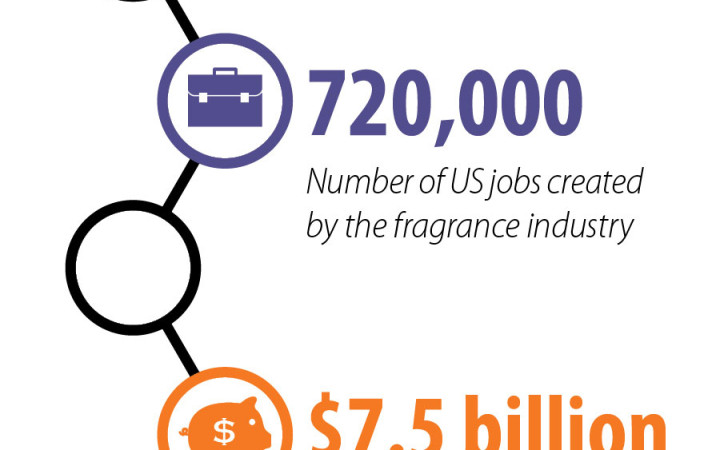 Making sense of the scent world. Click image to enlarge. Credits: design: Alex Hernsdorf; icons: freepik.com.
Making sense of the scent world. Click image to enlarge. Credits: design: Alex Hernsdorf; icons: freepik.com.
Extracting fragrances from natural sources
While we often experience the lively aromas of plants and flowers, bottling these scents was difficult and expensive before synthetic fragrances became available. To this day, perfumes made from natural materials are very costly. In New York City, the natural perfume store Lurk sells perfumes oils for $440 per ounce. On the West Coast, perfumer Mandy Aftel blends perfumes in her North Berkeley studio and sells them through her online company Aftelier for $720 per ounce. The classic Chanel No.5—widely considered to be one of the most expensive perfumes that uses synthetic fragrances—is $236 per ounce, almost cheap in comparison.
Natural perfumes are expensive because raw materials are costly to grow and transport. They are affected by unpredictable events such as weather, politics, and the economy, so they are not consistently available. Moreover, natural perfumes also require one very costly step: extracting oils from a plant.
There are three popular methods of extraction: expression, steam distillation, and solvent extraction. In expression, a press pushes out oils from the plant. This technique is commonly used for citrus oils. In steam distillation, steam is sent through the plant material. Plant oils have high boiling points, but they are volatile in the presence of steam. The steam carries the oils away from the plant material into a separate container, where the steam condenses. Plant oils do not mix with water, so they sit on the surface or fall to the bottom depending on their weight. Because of this natural separation, it is easy to remove plant oils from the water. In solvent extraction, the plant material is mixed with a solvent like hexane or ethanol. Removing the solvent leaves behind a thick, colorful mixture called concrete, which contains the oils as well as waxes and fats. Ethyl alcohol is used to remove most of the waxy substances and pigments from the concrete, leaving behind the oils.
In addition to their costly extraction process, natural fragrances are often more sensitive than their synthetic counterparts. Molecules in natural oils may degrade when they are added to cosmetics or household cleaners, which often contain harsh chemicals like detergents. Because these products make up a significant portion of the fragrance industry’s revenue, companies need fragrances that can be reliably used in these household goods, something that is difficult with natural compounds but much simpler for synthetics. Because of these factors and more, fragrance companies have developed a complicated and rigorous method for finding synthetic versions of natural fragrances.
The science of synthetic fragrances
Natural oils contain hundreds of molecules, but only a few molecules—called character-impact molecules—are responsible for the distinctive scent we associate with a particular material. The other molecules in natural oils are fixatives (heavy odorless molecules that keep other molecules in the oil from evaporating too quickly) and other odorants (which, smelled in isolation, do not remind you of the source material). To make synthetic fragrances, chemists analyze natural oils to detect the character-impact molecules, and then synthesize these molecules in the laboratory.
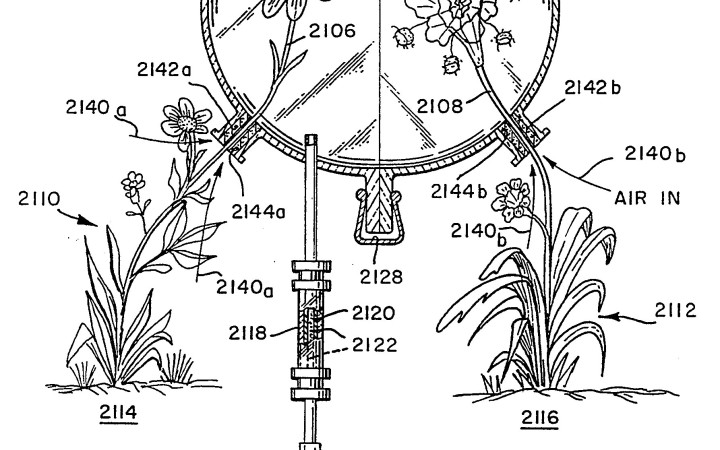 Headspace technology enables accurate identification of the fragrant molecules in a flower's aroma. Credit: U.S. Pat. no. 5,269,169 Fig A.
Headspace technology enables accurate identification of the fragrant molecules in a flower's aroma. Credit: U.S. Pat. no. 5,269,169 Fig A.
When a plant is too rare or too expensive to extract natural oils for study, scientists use headspace technology, a method for capturing the odors surrounding an object, like a smell-based camera. Headspace technology uses a bell jar-like object to trap the air surrounding the source of the scent, and the odorants are sucked out of the air and collected. They are then taken to a laboratory for analysis so the smell can be recreated as a synthetic fragrance. Because headspace technology grabs the air in a space, it can also be used to re-create the smell of places like tea houses or libraries.
Sometimes, fragrance companies search for new odorants to replace character-impact molecules. Like natural oils, character-impact molecules may break down when they are added to household cleaners, and even if these molecules are robust when added to products, they may be expensive to synthesize. As a result, chemists look for other odorants that produce a similar smell to the character-impact molecule but are more stable and cheaper to produce. Odorants with similar chemical structures often smell alike, so chemists start their search by slightly modifying the molecule’s structure. This process of making new odorants to replace naturally occurring ones can lead to new fragrances that have unique and enticing scents not necessarily found in nature. Galaxolide, a synthetic musk, is one such example. Unlike the warm, complex, and animalistic smell of natural musk, galaxolide has a soft sweet scent—one that is best described as “clean.” As a result, it is a popular addition to soaps, air fresheners, and laundry detergents.
While organic chemistry has led to significant advances in fragrance production, harnessing the chemical power of biological systems holds promise for producing the scents of the future. Scientists already use genetic engineering to produce drugs and fuel molecules, and some are beginning to use it to make synthetic fragrances as well.
The future: engineering microbial fragrance factories
Microorganisms naturally produce valuable chemicals that are used to make drugs, biofuels and even fragrances, but their normal yield is miniscule compared to industrial needs. To turn these bio-workshops into factories that churn out prized chemicals, scientists use metabolic engineering to manipulate the metabolic pathways in an organism. A metabolic pathway is a sequence of reactions that use enzymes to convert one compound into another. All the metabolic pathways in an organism form a complex network, which is similar to a street map in a big city. The goal of metabolic engineering is to increase the traffic through a particular pathway. Scientists close down alternative pathways and remove obstacles in the desired pathway using genetic engineering. This process is similar to a city putting up roadblocks on some streets and removing traffic lights on others in order to force cars along a particular route and toward a single destination.
Metabolic engineering is heavily used in the quest to make biofuels from plant material. Many biofuel molecules are derived from energy-rich fatty acids. “What you know as biodiesel is basically a mixture of fatty acid methyl esters,” says Harry Beller, director of the Biofuels Pathways Group at the Joint BioEnergy Institute and a senior scientist at the Lawrence Berkeley Laboratory.
But as it turns out, some fragrance molecules also stem from fatty acids. As a result, biofuel researchers sometimes uncover ways of engineering microorganisms to make fragrance chemicals. Beller, for example, originally had plans to make alkenes, compounds that are derived from fatty acids and are found in petroleum. Scientists in his research group engineered E. coli to make large quantities of fatty acids and thereby make lots of alkenes. However, an unforeseen byproduct of this approach changed the course of Beller’s research.
The engineered bacteria made unusually high amounts of methyl ketones, another derivative of fatty acids, and many of the genetic modifications the scientists made to the E.coli resulted in higher and higher levels of methyl ketones. “When we were trying to produce more of the target alkenes, we ended up producing more methyl ketones,” Beller says. “It was an unexpected finding.”
It was a welcome discovery for fragrance companies, however, because methyl ketones are highly-valued odorants used in both fragrances and flavorings. Furthermore, their scents vary depending on their chemical makeup. “Chain length is a very important factor in determining the taste or odor of methyl ketones,” says Beller, referring to variations in the number of carbon atoms in the chemical structure. Often, they are used to flavor cheese, but Beller’s group makes methyl ketones that smell of citrus and could be used in insect repellant.
At the same time, these methyl ketones are potential biofuels. They have a high cetane number—a measure of how quickly diesel fuel combusts. In the United States, a chemical must have a cetane number greater than 40 before it can be used as diesel fuel; the methyl ketones made by Beller’s E. coli have a cetane number in the mid-50s. Considering the potential utility of methyl ketones as both fragrance and fuel compounds, Beller and his group are now focusing on making these chemicals rather than alkenes.
Fragrance companies are already speaking with Beller about his group’s technology. “We’ve had quite a bit of interest from fragrance companies because this is not the typical way to make methyl ketones,” he says. “Traditionally, they use coconut milk and other types of milk solids and feed them to fungi.” Beller’s engineered, methyl ketone-producing bacteria are not only a new technology for fragrance companies, they may also be a more efficient one. “I think we have higher yields and faster production than what they are used to,” says Beller.
Beller is advocating a similar strategy for companies working to produce biofuels, who could sell highly sought-after chemicals like methyl ketones for other uses. “There’s not very much of a margin in the biofuels industry. Especially with the price of oil dropping, it’s really hard to compete with fossil fuels. So, producing high-value chemicals that can also serve as biofuels is actually a pretty good strategy,” says Beller.
Like Baur musk, Beller’s methyl ketones were a chance discovery towards making fragrance chemicals. Some scientists, however, are deliberately engineering microorganisms to do this. At the 2014 International Genetically Engineered Machine (iGEM) competition for undergraduate students, at least six groups experimented with ways to engineer microorganisms to make fragrance molecules.
Different groups focused on different fragrance molecules, reflecting the capability of microorganisms to make a variety of fragrances. Fragrances included hexanal, a compound that smells like fresh-cut grass; geraniol and beta-ionone, compounds found in rose oil; and terpenes, a diverse class of compounds that contain odorants like floral linalool and soothing menthol. Students from the University of Kent even performed a cost analysis to determine if their engineered E. coli could compete with technology currently used by fragrance companies to make zingiberene, the characteristic compound in ginger oil. Although the zingiberene from their E. coli was more expensive, their project shows the growing interest in using genetic engineering for making fragrances on an industrial scale.
The art of perfumery
For all the science involved in creating a particular smell, there has always been a strong dose of artistry as well. The perfumer, charged with delicately balancing the complex components of any smell, must combine many scents into an evocative perfume. Part chemist and part artist, perfumers work in labs with shelves stocked with hundreds of vials of fragrances.
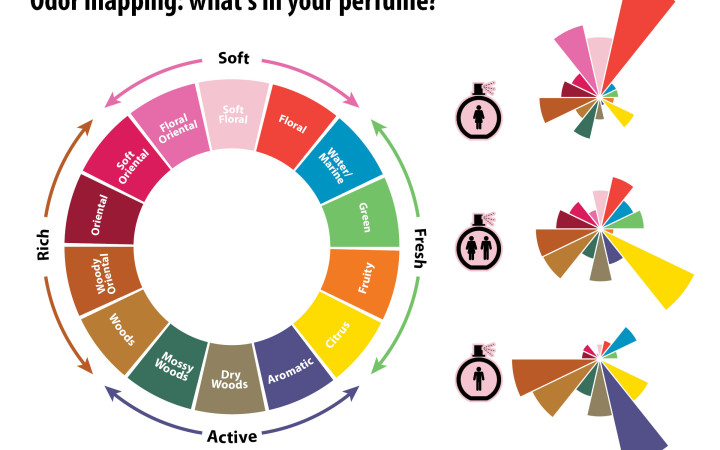 Odor mapping: what's in your perfume? Click image to enlarge. Left: A polar representation of the major scents that contribute to a perfume's overall aroma. Originally proposed in Michael Edwards' Fragrances of the World, this odor mapping has since received support from research in cognitive psychology. Right: the relative distribution of each scent group in women's, unisex, and men's perfumes. Credits: design: Alex Hernsdorf; perfume bottle: freepik.com.
Odor mapping: what's in your perfume? Click image to enlarge. Left: A polar representation of the major scents that contribute to a perfume's overall aroma. Originally proposed in Michael Edwards' Fragrances of the World, this odor mapping has since received support from research in cognitive psychology. Right: the relative distribution of each scent group in women's, unisex, and men's perfumes. Credits: design: Alex Hernsdorf; perfume bottle: freepik.com.
Perfumes are elaborate mixtures. They contain notes, or layers, of smells, because some odorants evaporate faster than others. Perfumers use this to their advantage to create a multi-faceted experience in a perfume. Top notes smell fresh and even sharp, evaporating within 15 minutes of putting on the perfume—just long enough to grab the wearer’s attention. Next come the mellow heart notes, the center of the perfume, which last for three to four hours. These scents are what most people remember about the perfume. Finally, the rich base notes balance the rest of the perfume and last for five to six hours.
The perfume Elle by Yves Saint Laurent, for example, is described as “a fragrance for an unpredictable and unique woman, in tune with her every desire.” The perfumers who designed Elle used woody and floral scents to convey a balance of strength and femininity. For the top notes, they chose lemony sweet citron and delicate lychee. The heart notes are peony, jasmine, and pink berries. Peony and pink berries are lively and vibrant, while jasmine is light and sensual. Finally, vetiver, patchouli, and ambrette make up the base notes. Combined together, vetiver and patchouli evoke sexiness whereas ambrette adds warmth.
A perfume’s complex structure can also be used to evoke a fleeting experience like the memory of a snowy day. It takes a skilled perfumer like Christopher Brosius to blend basic scents into such a perfume. Brosius owns I Hate Perfume, a store in Brooklyn, New York, where he makes perfumes like “Burning Leaves,” “In the Library,” and “Memory of Kindness.” He started the company after spending years as a taxi driver, carting people around in cabs and smelling their all-too-common perfumes.
In his words, a “perfume is a veil that reveals the soul.” This includes all the complex smells that are a part of life, like the smell of burning leaves or musty books. It can also be smells we associate with loved ones—“Memory of Kindness” smells like tomato vines, a tribute to Brosius’s kind aunt and her tomato garden.
Whether a perfume is designed to make you feel sexy, clean, or nostalgic, hidden behind its notes and scents is ever-changing science. Given how common fragrances are in our lives—from shampoos to detergents to makeup—we often forget about the ingenuity and work that went into making them. So the next time you wash your hair or pull a fresh load of laundry from the dryer, take a moment to reflect on the evolving process of fragrance making.
This article is part of the Spring 2015 issue.



