
You can follow Cindy on Twitter @xincindywang
--
It was March of 1942. As the last of New Haven’s chilly winter finally melted away, 33-year-old Anne Sheafe Miller lay in the hospital, dying of a streptococcal infection. A miscarriage had led to the stubborn illness, which left her slipping in and out of consciousness for four weeks as her temperature hovered between 103-106˚F. Her doctors put her through the entire battery of known treatments—blood transfusions, surgery, sulfa drugs—all to no avail. Hope was diminishing, and fast.
One final option remained. By chance, a fellow doctor at the New Haven Hospital had a close friend who worked at the pharmaceutical company Merck researching an experimental drug called penicillin. The compound, which came from a mold called Penicillium notatum, was discovered 15 years earlier when Scottish scientist Alexander Fleming had absent-mindedly left plates of Staphylococcus bacteria on his bench before going on vacation. When he returned, he found mold growing on one of his plates, but, amazingly, no bacteria grew around the mold. From this mold, he extracted and purified the substance we now know as penicillin.
By the time Anne got sick, penicillin’s therapeutic properties had been tested in a few mice and even fewer humans, all to mixed results. With their patient already at the brink of death, however, Anne’s doctors figured she had nothing to lose. Several phone calls to Merck convinced them to quickly produce a small quantity of penicillin for Anne, which was flown into New Haven and delivered to the hospital by a state trooper.
What happened next was nothing short of a miracle. The following morning, her fever broke and her temperature returned to normal. The day after, her appetite came back and she was soon eating full meals. Just a few days later, she was discharged, and she lived a full life until her death in 1999 at age 90. Penicillin afforded her over five more decades of life.
Fast-forward to present day: Anne’s story is no longer unique. With millions of lives saved, penicillin and other antibiotics are now stalwarts of our medicinal repertoire, turning what were once deadly illnesses into mere inconveniences. That magical panacea, however, is losing its touch. Bacteria are a veritable testament to the power of evolution; once exposed to an antibiotic, the susceptible bacteria die, and the infection heals. But every once in a while, a few stubborn bugs survive the treatment. These are the resistant ones—invincible towards antibiotics, they retain the means to kill.
Antibiotic resistance is now responsible for over 23,000 deaths each year in the United States, a number that only continues to rise. Without proper action, we could quickly degenerate to pre-antibiotic days, when a routine childbirth could kill an otherwise healthy patient like Anne. Thankfully, a large number of dedicated scientists, including many at UC Berkeley, have devoted their research to understanding and combating antibiotic resistance. Their dedication renews hope that solutions may yet be found.
A cat-and-mouse game
Our story of antibiotic resistance begins with the bacteria Staphylococcus aureus (S. aureus), the root of the common staph infection. The first penicillin-resistant strain was reported in a patient in 1946, only four years after Anne’s miraculous recovery proved penicillin’s efficacy. Luckily, the chemical structure of penicillin had recently been solved, revealing at its heart a four-atom ring called a ß-lactam. By altering the atoms attached to the ring, scientists found that they could create new and more potent antibiotics.
 Credits: Design: Jo Downes, with modified elements from Cindy Wang
Credits: Design: Jo Downes, with modified elements from Cindy Wang
And so they did. The 1950s and 1960s saw a large influx of new antibiotics on the market, among them methicillin, introduced by the British chemical company Beecham in 1959. Its success against penicillin-resistant S. aureus made it a big hit among physicians—but not for long. A mere three years later, methicillin-resistant strains of S. aureus, or MRSA (pronounced mur-suh), struck Queen Mary’s Hospital for Children in a London suburb. Slowly, outbreaks spread throughout Europe, eventually reaching the United States with an outbreak at Boston City Hospital in 1968.
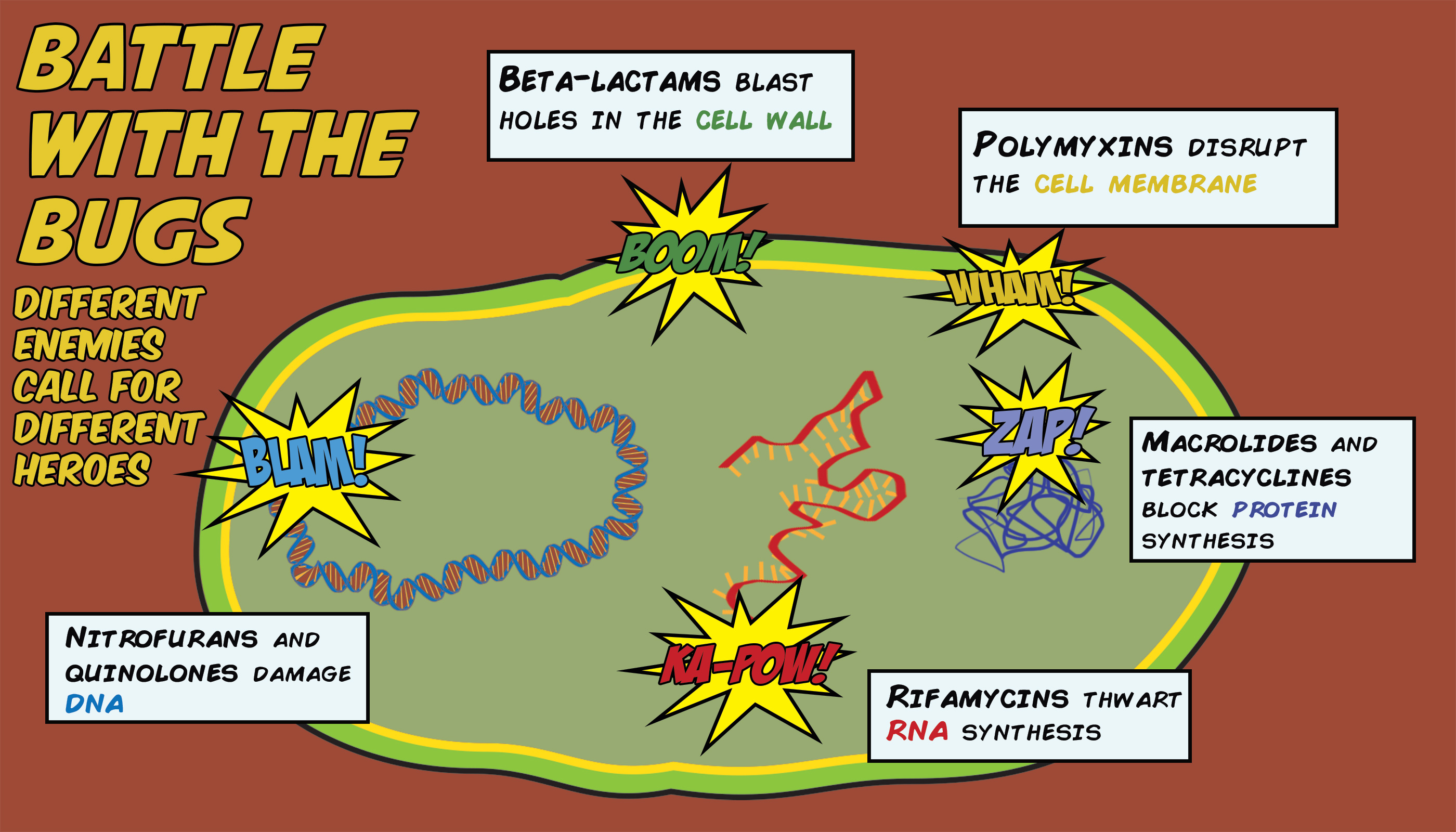
Penicillin, methicillin, and other ß-lactam antibiotics work by interfering with the cell’s ability to make new cell wall. All bacteria have a rigid cell wall that encapsulates their contents and provides structural support. That cell wall is composed of molecules of peptidoglycan, which are all linked to each other by an enzyme called transpeptidase. If the peptidoglycans are the bricks, then the transpeptidase is the bricklayer, arranging and locking the bricks into a highly organized structure. ß-lactams bind to and inactivate the transpeptidase, knocking out the bricklayer and with it any potential for structural remodeling. Without the ability to build new cell wall, bacteria cannot contain their continuously replicating insides, and they eventually burst at the seams.
MRSA’s resistance can be traced to an enzyme called ß-lactamase, which recognizes and breaks down the ß-lactam ring present in penicillin, methicillin, and other ß-lactam antibiotics. By changing the atoms around the ß-lactam ring, scientists created new antibiotics that could disguise themselves from existing ß-lactamases, ensuring their efficacy—but only until the bacteria would evolve to recognize the disguise.
Each time a bacterium replicates its genome for cell division, it makes a few random mistakes. Some mistakes have no effect, while others prove fatal for the bacterium. Sometimes, those mistakes hit the ß-lactamase gene jackpot, changing the ß-lactamase ever so slightly so that it can recognize and destroy the new antibiotic. Unfortunately for us, most bacteria replicate their genomes at least once an hour; S. aureus only takes 30 minutes to divide. With each new generation comes another opportunity for the ß-lactamase to evolve, so it doesn’t take very long for a newly resistant strain to develop.
The war against bacteria has always been a tense cat and mouse race to stay one step ahead of evolution. When it comes to ß-lactams, we’ve now nearly exhausted the scope of –illins, we’ve gone through five generations of the more powerful cephalasporins, and we’re now on the even more powerful carbapenems. Methicillin is no longer used or manufactured, but the name MRSA stuck, and it now refers to a multidrug-resistant S. aureus whose only effective ß-lactam adversary is a fifth-generation cephalosporin that hasn’t yet been approved for use in the United States.
Thankfully, our arsenal of antibiotics extends beyond ß-lactams, and we have other drugs that can treat MRSA. These antibiotics work through other mechanisms: some inhibit DNA synthesis, others inhibit protein synthesis, while others target the cell membrane, all of which are necessary for the bacteria’s survival. Yet even with this diversity, bacteria continue to find ways to build resistance. They can break down the drugs, as ß-lactamases do, or change the drug targets so that the drugs can no longer act. They can block the drugs from getting in, or pump them out once they are inside. We’re smart, but the bugs are smarter, making it only a matter of time before these other drugs also lose their effectiveness against MRSA.
Meanwhile, MRSA only continues to spread. The first MRSA epidemics were contained within hospitals, as the bacteria spread easily through shared equipment. By the 1980s, however, outbreaks started appearing in the community, with epicenters pinpointed to parks and other public areas. Nowadays, the United States Centers for Disease Control and Prevention (CDC) estimates that 50% of people actually carry MRSA on their skin, usually without infection. This means that routine papercuts and pickup football games carry an unsettlingly high risk, as any open wound could give MRSA a gateway into the bloodstream. Of the over 100,000 cases of MRSA reported in the US each year, 20% result in death.
A rapid threat
On the other side of the globe, Mohammad Moydul Islam walks through the streets of Dhaka, Bangladesh, in a white lab coat. He wears a mask over his face, two layers of latex gloves over his hands, and carries a red bag with a large biohazard warning label. Curious passersby ask the masked man what he is up to. “I’m doing some serious work,” he replies. “You don’t have any protective equipment on – it’s best to stay away.”
He sees the uneasiness in their eyes and tries to allay their anxieties by smiling, but he realizes too late that his smile is hidden under his mask. Undeterred, he goes back about his business. He sweats profusely under his lab coat in Dhaka’s hundred-degree summer heat as he squats down next to a puddle outside the local hospital, dipping a swab into the seepage water.
Mohammad Moydul, known to his friends as Polash, is a research officer in the group of Dr. Mohammad Aminul Islam (no relation) at the Center for Food and Waterborne Diseases in the International Center for Diarrheal Disease Research, Bangladesh (ICDDRB). Polash is collecting wastewater samples from around Dhaka to test them for the presence of microorganisms that contain NDM-1, the gene encoding New Delhi Metallo-ß-lactamase-1. NDM-1 is no ordinary ß-lactamase: it’s actually a carbapenemase, which means that it can break down carbapenems, the most potent ß-lactam antibiotics in existence. Because carbapenems are immune to most ß-lactamases, they’re our last line of defense against drug-resistant bacteria. But carbapenems don’t stand a chance against NDM-1.
“It’s a huge concern,” Polash says repeatedly over the course of our interview, which he always follows with a sheepish grin, as if to allay the gravity of the situation. Polash already knows that NDM-1 lurks around Dhaka—his masters’ thesis research identified NDM-1-producing organisms in stool samples of diarrheal patients from around the city. He tested the NDM-1-positive bacteria against 15 different antibiotics, including a number of third-generation cephalosporins, the most potent drugs readily prescribed by local doctors. They were resistant to all but two, which doctors avoid because of their noxious side effects: colistin causes nerve and kidney damage, and tigecycline leads to lupus, hepatitis, tinnitus, and a myriad of other secondary disorders.
In the timeframe of antibiotic resistance, NDM-1 is just a baby. It was discovered in 2009 from a Swedish patient who had contracted a multidrug-resistant strain of Klebsiella pneumoniae during a trip to New Dehli. That said, it has already proved itself quite precocious. A year later, NDM-1 cases were reported in the United States, Japan, and England, while a wastewater study in New Delhi found NDM-1-positive bacteria in 30% of the samples tested. Only two years later, Polash’s study found NDM-1 in 9% of diarrheal patients in Dhaka, and his ongoing wastewater study could very well confirm NDM-1’s presence in the greater Dhaka community.
Bacteria are particularly good at acquiring and spreading resistance because they have the ability to transfer genes between one another, even between different species. In fact, many carbapenemases originated in bacteria that don’t cause disease and only found their way to disease-causing bacteria through these promiscuous gene-swapping habits. Resistance genes, including NDM-1, often appear in discrete, circular pieces of DNA called plasmids, which bacteria can easily pick up from other bacteria or from the environment. In addition to expressing the genes on the plasmid, the bacterium can also replicate the plasmid and, if the plasmid allows, directly incorporate plasmid genes into its own genome, ensuring that those genes pass onto the next generation and beyond.
Because of this, rampant antibiotic overuse and misuse is another key reason why resistance spreads so quickly. In poorer areas, antibiotics remain cheap and plentiful, making them obvious go-to drugs for even the most minor illnesses. The cost of one antibiotic pill in Bangladesh is equivalent to a U.S. dime. “[There are] no rules, so if you ask and if you pay the money to the vendor or the pharmacist, he will directly give [it to] you,” Polash told me.
 Polash Islam hunts for antibiotic-resistant bacteria in Dhaka,
Polash Islam hunts for antibiotic-resistant bacteria in Dhaka,
This lack of regulation presents a host of other problems. Without a proper diagnosis or dosage, the antibiotics could allow the resistant strains to dominate without actually solving the problem at hand. Furthermore, patients often do not take the antibiotics for the full course of treatment. “Regular people don’t know the consequences, so after two or three days they start to feel ok and stop [taking the antibiotic], but you have to finish the full course. If you stop before that, then eventually you will create resistant organisms within yourself,” Polash explains. Regulation has indeed proven effective in curbing resistance. In Sweden and Norway, two countries with highly standardized and socialized healthcare structures, less than 1% of Staph infections are due to MRSA. In the United States, that figure is a whopping 70%. In Bangledesh, the statistic is unknown. “If we want to prevent the spread of these multidrug-resistant organisms, then we need policies promoting the prudent use of antibiotics. That should be the topmost priority,” Polash urges.
Human antibiotic use, however, only comprises a fifth of the world’s total antibiotic consumption. The majority of antibiotic use actually occurs in agriculture, where the drugs are used to prevent and treat infections and reduce chances of food-borne illness. Low levels of antibiotics are also routinely added to livestock feed to promote animal growth. The reason why this works remains a mystery, but scientists speculate that antibiotics kill microbes in the animal gut that would otherwise compete with the animal for nutrients. Although antibiotic use in livestock has largely proven beneficial towards improving food production and safety, multidrug-resistant strains of bacteria have started finding their way into our food supply. An outbreak of drug-resistant Salmonella infected over 630 people in 30 U.S. states over the last year. The culprit? Chicken from Foster Farms, a company that, ironically, doesn’t use antibiotics for growth promotion.
On the produce side, antibiotics and antimicrobial pesticides are also commonly used to curb disease and increase yields. While this also leads to resistance, water or soil contamination actually poses a greater threat. “Antibiotic overuse in humans and animals means that resistant organisms could easily get into the water, and that water gets used on the vegetables,” explained Caitie Cook, a master’s student and researcher in Lee Riley’s lab at the UC Berkeley School of Public Health. Caitie spent last summer in Dhaka working alongside Polash hunting for drug-resistant bacteria on vegetables sold in markets around the Bangledesh capital. Although she has no conclusions yet, the prognosis is grim. A few years ago, another member of the Riley lab found drug-resistant bacteria on baby spinach bought from a supermarket in Berkeley. Dhaka, with its poorer sanitary conditions and fewer antibiotics use regulations, would likely have even higher levels of resistant bacteria.
It’s a huge concern.
Tip of the iceberg
Back at UC Berkeley, doctoral student Sheila Adams-Sapper, also in Lee Riley’s lab, checks in on her cultures of drug-resistant Klebsiella pneumoniae. These strains of Klebsiella harbor the deadly weapon KPC, or the Klebsiella pneumoniae carbapenemase, rendering them resistant to the most powerful ß-lactam antibiotics currently available. With a 50% death rate, KPC-producing Klebsiella has already claimed over 600 lives in the US alone.
The full story behind its resistance, however, isn’t as simple as having or not having the KPC gene. Many organisms that carry KPC actually initially test as being fully susceptible to carbapenems. Doctors start the patient on carbapenems, but after a few days of treatment, the patient doesn’t show any signs of getting better. Perplexed, more samples are taken, and the bugs are tested again. This time, they turn up resistant. What happened in between?
Sheila’s research attempts to answer that question. Surprisingly, she found that both the susceptible and resistant bacteria—before and after treatment—belonged to the same strain, meaning that they shared the same DNA, ruling out the notion that a few resistant mutants took over. In fact, both susceptible and resistant bacteria also produced the same levels of KPC, which also ruled out the notion that the gene was only activated in a few cells. Something else was allowing some of them to survive better than others.
That something else turned out to be porins, or pores on the bacterial surface through which molecules enter and exit. Sheila gave her KPC-producing Klebsiella lethal doses of carbapenems and found that the ones that survived were missing a large number of porins on their surface, limiting drug entry. This would keep the carbapenem levels inside the cell at a manageable level, allowing the carbapenemase to break down the drug without getting overwhelmed.
Without porins, the cell also has difficulty getting nutrients, and they grow extremely slowly. “You’d think that’d be a fitness disadvantage, but in this particular case, the killing disadvantage outweighs the ability to get nutrients,” Sheila reasons. “Somehow they shut down their metabolism enough so that even without enough nutrients, they can still persist.” This slow growth gives the bacteria another hidden advantage: most antibiotics target processes that contribute to cell growth, in the way that ß-lactams prevent cell wall synthesis. Without growth, these antibiotics have nothing to target.
Sheila’s research highlights the immense complexity behind bacterial metabolism and resistance mechanisms, drawing attention to how little we understand about the inner workings of the cell. But the complexity doesn’t end there—we’re just as ignorant in what happens outside the cell, a fact that Will Ludington knows all too well. As a microbial ecologist and a Bowes Fellow in the UC Berkeley Department of Molecular and Cell Biology, Will studies the microbiome, or the complex ecosystem of microbes that inhabit a living body.
These microbes coexist in our bodies in a mutually beneficial arrangement: we provide them with shelter, while they help carry out many of our bodily functions, including digestion. However, the broad-spectrum antibiotics typically prescribed to combat infections indiscriminately kill all bacteria, compromising the balanced microbiome ecosystem. “That whole approach makes you vulnerable because you’re killing off what you already have in your system,” Will explains. The human microbiome is incredibly resilient and can oftentimes restore that balance on its own. We are often too quick, however, to jump on antibiotics as an easy solution. After a few days, as we begin to feel better, we’re likely to give antibiotics all the credit for restoring our health, when in fact our own microbiomes likely contributed just as much, if not more, to our recovery.
Will hopes to develop a more quantitative model for understanding bacterial networks and populations, especially as they respond to changes in the environment. Previously, Will had spent five years as a field ecologist on the small Pacific island of Palmyra Atoll, but he realized that he could only learn so much from diligently cataloging the island’s various species. That’s when he decided to turn to microbes. “In island ecology, they’re measuring things like 100-year data sets that only scratch the surface. In the bacterial system…you can perturb the environment—you don’t have to get chainsaws or reciprocating saw out in the middle of the forest and cut down a bunch of trees.”
Will’s ultimate goal is to create targeted therapeutics that would only knock out the particular strain of bacteria causing the infection. “If we could go in and say, ‘I just want to kill off this one pathogen that’s causing this problem and not hurt the other ones,’ then you don’t have this vulnerable open system,” he muses. This goal, Will admits, is a bit of a pipe dream, but we’re inching closer. Already, DNA-based rapid diagnostics can identify the disease-causing bacterial strain in just a few hours, solving the first part of the problem. The challenge now lies in finding a way to selectively knock out that particular strain.
 Credits: Design: Jo Downes
Credits: Design: Jo Downes
A shift towards innovation
Historically, the search for new antibiotics has always involved large-scale screens of chemical libraries: make millions of compounds, test them against many different types of bacteria, and see if any of the compounds can kill. These brute-force drug screens, however, are the molecular counterparts to finding needles in haystacks. They require time, money, manpower, and many more resources—all for a massive fishing expedition that could yield no fish.
It’s no surprise, then, that most large pharmaceutical companies have abandoned antibiotics research in favor of more lucrative ventures. The economics are simple. While a cancer patient might spend hundreds of thousands of dollars over several years on drugs, a patient suffering a bacterial infection might spend only $100 on a two-week course of antibiotics. Furthermore, the newest, most potent antibiotics would largely be last-resort medications, reserved for only the most desperate cases. The inevitability of resistance also gives new drugs just a few years of efficacy. Accordingly, the number of new antibiotics approved for use in the US has fallen from 19 in 1984 to four in 2004. Between 2010 and 2012, only one new antibiotic came into the market.
These facts underscore the need for innovation in antibiotics discovery. One approach is to screen for compounds that interfere with novel targets such as pieces of bacterial RNA called riboswitches. Riboswitches toggle genes on and off in response to binding small molecules. “The genes controlled by riboswitches are usually important for cellular metabolism, so drugs that inhibit normal riboswitch function may have antibacterial activity,” explained Scott Hickey, a graduate student in Ming Hammond’s lab in the UC Berkeley Department of Chemistry.
Riboswitch-targeting antibiotics have already been identified. The natural product roseoflavin, for instance, targets a riboswitch in the riboflavin (vitamin B2) pathway. Recently, Scott developed a method to quickly screen libraries of compounds for riboswitch targeting. Although Scott’s research focused on one particular riboswitch, his method could be adapted for the thousands of different riboswitch sequences that exist in bacterial genomes, paving the way for the rapid discovery of new antibiotics.
 Credits: Design: Jo Downes
Credits: Design: Jo Downes
Other new approaches avoid the needle in a haystack drug screen altogether, focusing instead on predicting potentially effective compounds. One strategy draws upon the resilience of the human microbiome, which already has its own ways of keeping specific bacterial populations in check. Could we leverage those mechanisms to find natural antibiotics?
The answer is yes. Researchers in Michael Fischbach’s lab at UC San Francisco created a computer program that combed the genomes of bacteria in the human microbiome to look for gene clusters that might make small drug-like molecules. One gene cluster, from the vaginal bacteria Lactobacillus gasseri, made a molecule called lactocillin. When they exposed other bacteria—including S. aureus, of MRSA fame—to lactocillin, the bacteria died, confirming lactocillin’s antibiotic properties. Intriguingly, the compound closely resembles another antibiotic that the pharmaceutical company Novartis is currently testing in clinical trials, validating the potential of their gene-mining approach.
Such compound-based approaches, however, still don’t tackle the ultimate challenge of creating therapeutics that target one specific bacterial strain. Furthermore, the small molecule drugs that these methods yield are still prone to the existing mechanisms of resistance. Having a truly selective therapeutic requires targeting something that’s unique to each bacterial strain. For that, we can turn to DNA.
Just this September, researchers in Timothy Lu’s lab at the Massachusetts Institute of Technology engineered a way to selectively kill bacteria harboring antibiotic resistance genes such as NDM-1. To do this, they applied the CRISPR/Cas genome editing system, a technology pioneered by Jennifer Doudna’s group at UC Berkeley that directs the DNA-cutting enzyme Cas9 towards a particular gene sequence. Directing Cas9 towards NDM-1 cuts the resistance gene out of bacteria, restoring their susceptibility to carbapenems. They also demonstrated that they could customize these cuts for a specific bacterial strain to kill only that strain in a mix of many.
These new approaches hold much promise in combating antibiotic resistance, but they have a long way to go before they can be translated into actual therapeutics. Fortunately, policymakers are also recognizing the need for action. The President’s Commission on Science and Technology, comprised of the nation’s leading academics, recently released a 78-page report outlining recommendations towards curbing antibiotic resistance, including tighter surveillance of antibiotic use, increased funding for academic research, and more incentives for pharmaceutical companies to conduct antibiotics research. Meanwhile, citizens in the United Kingdom voted for antibiotics as the focus of the government’s Longitude Prize 2014, a £10-million prize that will be awarded for innovative solutions towards creating, according the prize website, “a cost-effective, accurate, rapid, and easy-to-use test for bacterial infections that will allow health professionals worldwide to administer the right antibiotics at the right time.” With this support and attention, we can remain optimistic that good solutions are on their way.
But until then, you better watch out, because every cut could very well be your last.
This article is part of the Fall 2014 issue.



