Contrary to popular belief, humans continually produce new brain cells. Every day around ten thousand new neurons are born. Exactly how this occurs is unknown, but some researchers hypothesize that once born, young neurons are important for memory formation. The neurons appear when a population of brain cells, called neural stem cells, divide and differentiate, or become specialized for specific functions. Gaining control of the cellular mechanisms involved will help elucidate how differentiation is regulated.
 Optogenetics give researchers fine-tuned control over neuronal activity
Optogenetics give researchers fine-tuned control over neuronal activity
Recent work in the Schaffer lab at the University of California, Berkeley has begun to make gaining control a reality. Neural stem cells differentiate based on information from cell signaling pathways. These pathways are initiated when a cue, usually in the form of a protein or small molecule, interacts with a receptor on a cell. The receptor then activates other proteins within the cell, leading to a cascade of activated proteins that produce the cell’s response (essentially a protein-mediated version of the classic “telephone game”). Schaffer’s lab has begun to co-opt the pathway that responds to the “make a new neuron” cue with hopes of controlling neuron production on demand. To develop an optogenetic toolkit, Bugaj and Schaffer wanted to leverage proteins that naturally respond to light, so they turned to an intrinsically light sensitive organism: the common lab plant Arabidopsis. They found a protein that “plays a significant role in…the ability of the plant to respond to blue light and control timing of flowering for the plant,” explains Schaffer. “So, it was a good starting point to utilize to engineer light sensitivity into novel proteins.” Bugaj then appended the blue-light sensitive portion of this plant protein to a receptor for a signaling pathway with a major role in neural stem cell differentiation. Previous studies have shown that causing the receptor to form clusters (a process called oligomerization) could activate the pathway. Incredibly, when Bugaj shined blue light on cells expressing the engineered receptor, the first thing he noticed were clusters forming. As expected, this clustering activated the pathway and the game of telephone within the cell began, only ceasing when the light was turned off. “It’s like you have a remote control over signaling,” Bugaj said.
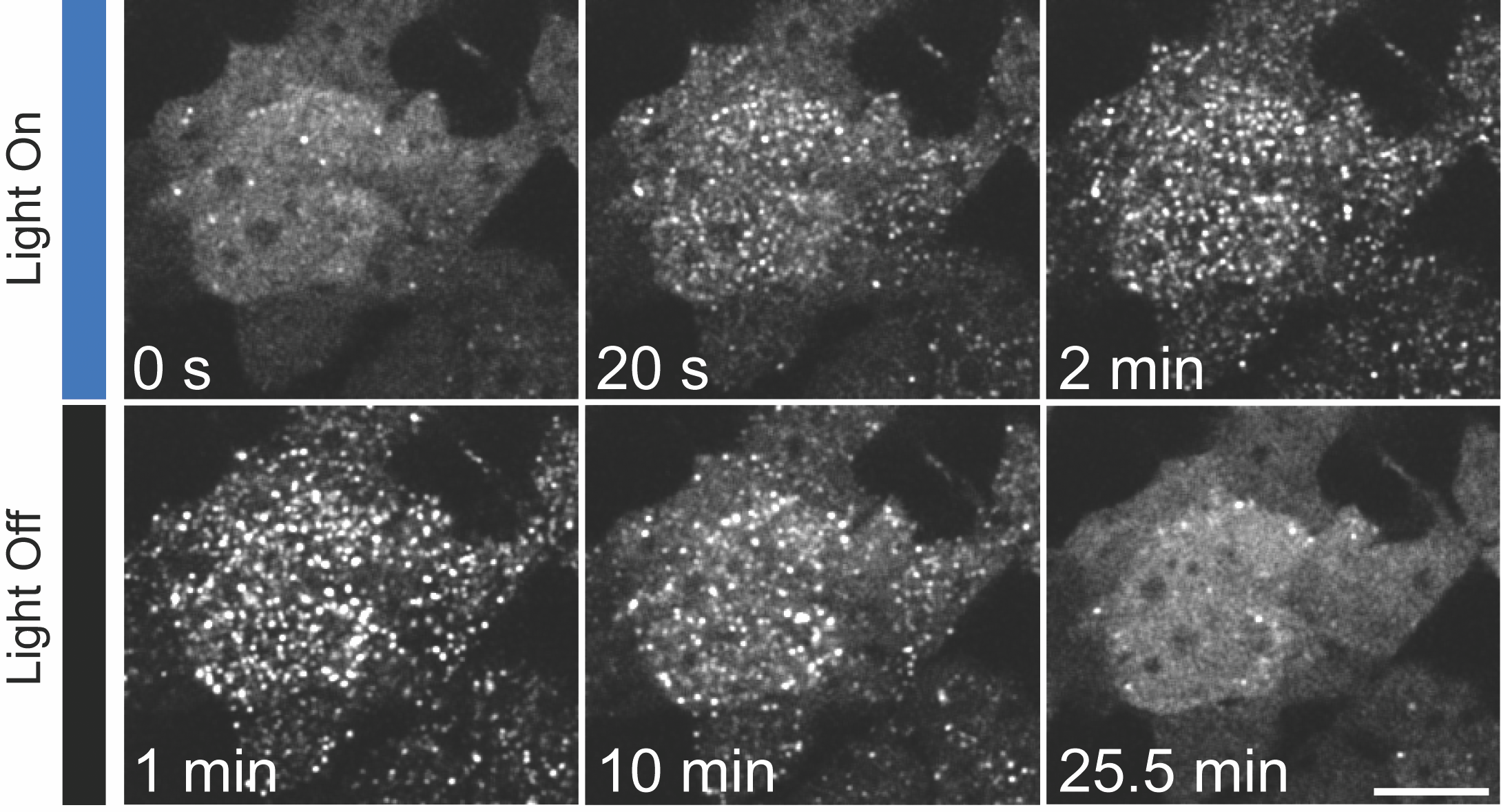
 When blue light illuminates a cell expressing optogenetically engineered proteins, fluorescent receptors on its membrane appear to form clusters, or bright dots. This process reverses when the light turns off. Such clustering is known to activate a signaling pathway that causes the cell to become a neuron. Credit: Lukasz Bugaj
When blue light illuminates a cell expressing optogenetically engineered proteins, fluorescent receptors on its membrane appear to form clusters, or bright dots. This process reverses when the light turns off. Such clustering is known to activate a signaling pathway that causes the cell to become a neuron. Credit: Lukasz Bugaj
While this was the first demonstration that controlling intracellular signaling pathways with light was possible, Bugaj wanted to know: “Can you also regulate other signaling pathways or did we just get really lucky?” To find out, he attached the light sensor to another important signaling molecule in neurogenesis, RhoA, which controls a cell’s shape and motility. After shining blue light on these engineered cells, the researchers saw a robust and reversible contraction of the cell membrane.
In light of these results, there is potential to have fine-tuned control over a wide range of oligomerization-based signaling pathways. Indeed, after their paper was published, so many scientists wanted to use the engineered protein to investigate other signaling pathways that Schaffer submitted a copy of its DNA sequence to an online distributor to keep up with demand. Since developing the tool, Schaffer and his students have begun to answer more complex questions, like “what’s the threshold of signaling for a cell to commit to turning into a neuron?” In addition, Bugaj hopes to “expand the palette” of colored light that can be used to control signaling. “With light you can easily pattern gradients in all sorts of fashions, so really the questions you can ask are limitless,” he emphasizes. “It’s very recently that people have begun to appreciate that maybe we can actually do this, maybe we can have such precise control.” This precise control may ultimately help scientists determine the mechanisms that generate new neurons, and in turn the mental processes they underlie.
This article is part of the Fall 2013 issue.



