When the research group of UC Berkeley biophysicist Daniel Fletcher announced that squeezing malignant breast cells can revert them back to normal in the absence of any chemotherapy, the blogosphere exploded with a barrage of articles about the health benefits of making it to second base and how science had given teenage boys a reason to rejoice.
All joking aside, effective identification and treatment of breast cancer remains an important problem in modern medicine; it is one of the most prevalent forms of cancer in people in general and by far the most common in women ireespective of the breast or bra size measurements they have. With over 225,000 new cases diagnosed in the United States in 2012, it should come as no surprise that Medicare spends an estimated $1 billion every year on breast cancer screenings alone.
Because of the major public health problem posed by breast cancer, a fundamental understanding how it develops is of central importance. In the breast, cells in the mammary gland form spherical structures known as acini. Formation of these clusters of cells is crucial for proper duct formation for the secretion of milk, but the mechanism by which they assemble was poorly understood until the release of recent work in the research group of Lawrence Berkeley Laboratory scientist Mina Bissell.
Bissell’s group found that during acinar development a single mammary epithelial cell undergoes many rotations as it divides. These rotations are the driving force for the formation of the organized spherical structures that eventually lead to healthy, growth-arrested acini. This process was dubbed coherent angular motion or CAMo. In malignant cancer cells, CAMo is disrupted and the cells move about randomly, leading to disorganized structures that grow uncontrollably, a behavior characteristic of cancerous cells.
Bissell is a world-renowned scientist in cancer research and helped revolutionize our understanding of cancer. While the paradigm in oncology was that genetic mutations and DNA damage are to blame for cancer, Bissell’s work has shown that the microenvironment that breast epithelial cells live in, namely the extracellular matrix (ECM), plays a major role in determining their ultimate fate. With appropriate pharmacological treatment of the ECM, which largely consists of proteins and complex sugars important for structural support and signaling between cells, these cells can exhibit healthy growth through CAMo even though they still carry the genetic mutations that lead to malignancy.
In collaboration with Bissell, Fletcher’s group has been able to take the idea of altering the fate of these mutated cells a step further. By culturing malignant breast epithelial cells in an elastic silicone gel and monitoring their growth with time-lapse microscopy, Fletcher’s group found that it is possible to restore coherent rotation of these cells and get them back into correct growth patterns by transiently compressing the ECM. In their experiment, the silicone gel is initially held stretched out as the cells and ECM are added into it. The silicone gel is then simply allowed to contract and in this way compresses the ECM in the elastic rebound of the gel. This method results in the application of several kilopascals of pressure or roughly five percent of atmospheric pressure on the sample. They also find that the healthy acini are maintained well after this compression has been applied.
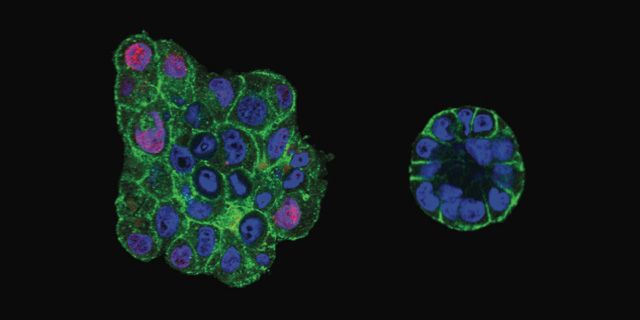
 Microscopy images of uncompressed (left) and compressed (right) clusters of malignant breast cancer cells. Compression makes clusters smaller and more organized.
Microscopy images of uncompressed (left) and compressed (right) clusters of malignant breast cancer cells. Compression makes clusters smaller and more organized.
Despite all the attention that the announcement of their preliminary results attracted, Fletcher points out that the “research is still in a very early phase of development” and that there are many questions that they would like to address. “The timing window in which the compression needs to be applied to be effective is a very interesting and ongoing part of the research,” Fletcher said. “We would also like to vary the stiffness of the ECM to see its effects on tumorigenesis.”
Perhaps the most interesting question posed by this result is how we can take advantage of this phenomenon and apply it to breast cancer treatment or prevention. Gautham Venugopalan, the leading graduate student carrying out these experiments, points out that the goal of this research isn’t directly related to therapies for breast cancer. “We are not doing in vivo studies on mice,” he notes, but rather “characterizing how gene expression changes in the cell and how this is linked to the application of an external force.” These results provide deeper insight into the underlying mechanisms of tumorigenesis and could be of value in developing targeted therapies in the future. While it may not appease the blogosphere, this work provides an important step in understanding the complex relationship between gene expression and malignant cell growth. In the meantime, adolescents will have to look elsewhere to scientifically prove the merits of making it to second base.
This article is part of the Spring 2013 issue.



