Solar energy has become a hot topic as public awareness of climate change and environmental degradation grow alongside political promises of a “green” rebirth of the struggling US economy. With the mere use of tools like utilitysavingexpert.com, one can truly understand how much electricity is wasted annually. Solar cells, or photovoltaics, are able to convert the energy provided by the incoming rays of the sun directly to electricity. Given this ability to harness the vast and virtually untapped resource of solar radiation and turn it into useable power, photovoltaics seem poised to form the cornerstone of an evolving clean energy policy. However, ask any economist and you will discover that the costs associated with the materials, manufacturing, and installation of solar cells seriously limit their economic feasibility and necessitate the use of heavy government subsidies in order to keep the photovoltaic industry afloat. UC Berkeley professor Peidong Yang, along with his former student Erik Garnett, hopes to change this trend by using cutting-edge nanometer-scale fabrication techniques to significantly reduce the materials costs of silicon-based photovoltaic cells.
Most of the solar panels seen on buildings and homes today are made of silicon, the abundant and ubiquitous semiconductor that is the foundation of the modern computing industry. In fact, silicon solar panels make up about 70-80 percent of the worldwide market for photovoltaics. In a conventional cell, flat regions of electron-rich and electron-scarce silicon sit next to each other, forming what is known as a “planar p-n junction” (the p and n stand for positive and negative, respectively). As light from the sun strikes the cell perpendicular to the plane of the junction, the silicon must serve two purposes to allow efficient operation. First, in order to maximize the conversion of the sun’s energy to electricity, the cell must absorb as many of the incident photons as possible. Since silicon does not absorb light as well as some other semiconductors, a relatively thick layer is needed (imagine light shining through a thin sheet of paper: the thicker the stack of pages, the less light makes it through). Second, the silicon must allow electric charges to move effectively from the p-n junction to the surface of the cell where they can be connected to a circuit and used. However, there is some resistance to this movement caused by impurities in the material and thus, the thinner and purer the layer of silicon, the more effectively it can transport electrons and electron vacancies (known as “holes”) to their destination.
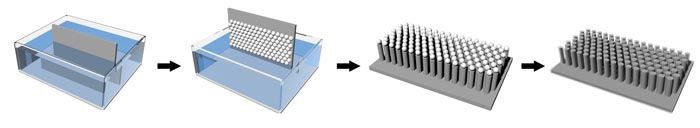
 This schematic shows the fabrication process for the silicon anowires. A silicon wafer is first dipped into a liquid suspension of silica beads. When the wafer is removed from the liquid, the highly uniform beads—like tiny grains of sand—stick to the surface of the wafer in an ordered, closely packed pattern. The exposed areas of silicon are then etched away, leaving behind straight pillars. Finally, the silica beads are removed using hydrofluoric acid. The silicon nanowires remain intact, ready to be built into a solar cell. Credit: Erik Garnett; Peidong Yang; Nano Letters. 2010, 10, 1082-1087. Copyright 2010 American Chemical Society
This schematic shows the fabrication process for the silicon anowires. A silicon wafer is first dipped into a liquid suspension of silica beads. When the wafer is removed from the liquid, the highly uniform beads—like tiny grains of sand—stick to the surface of the wafer in an ordered, closely packed pattern. The exposed areas of silicon are then etched away, leaving behind straight pillars. Finally, the silica beads are removed using hydrofluoric acid. The silicon nanowires remain intact, ready to be built into a solar cell. Credit: Erik Garnett; Peidong Yang; Nano Letters. 2010, 10, 1082-1087. Copyright 2010 American Chemical Society
In a conventional planar cell, both absorption of light and movement of charge carriers occur in the same direction. The processes thus compete against one another—due to the thickness of the layer needed for adequate absorption, very pure silicon must be used to lower the resistance and allow the cell to operate efficiently. This is bad news in an industry where cost is everything: not only does one need a lot of silicon to absorb the right amount of light but that silicon must also be highly pure, which makes it drastically more expensive. Yang and Garnett’s solution is to rethink the geometry of the solar cell so that absorption and charge movement happen in different directions. “By working with one-dimensional nanostructures, we can orthogonalize the light absorption and the charge separation,” says professor Yang. “This is an effective way of improving the movement of electric charge in the solar cell.”
Working with Dr. Garnett, Yang’s research group has developed a simple method for fabricating highly uniform silicon nanowires that could make solar cells significantly cheaper. This novel process makes wires that are typically about 400 nanometers in diameter—about 1000 times thinner than the average human hair—that stand up vertically like a nanoscale bed of nails. When a photovoltaic cell is made from a surface covered in these miniature pillars, the p-n junction, instead of being a flat plane, is now in the shape of a cylinder centered on the axis of each individual nanowire. In this “radial p-n junction,” light is absorbed along the length of the wire—typically many micrometers, providing ample absorption—while charges are moved to the surface across its width. Since the electrons and holes must only move a few hundred nanometers in order to be used, there is less resistance due to impurities. Thus, the same relative level of performance can be achieved using less pure and significantly cheaper silicon, lowering the overall cost of the solar cell.
 An image taken using a scanning electron microscope (left panel) shows the effectiveness of Yang and Garnett’s growth technique. The vertically aligned pillars are each approximately 400 nanometers in diameter and five micrometers in height. The excellent periodicity of the pillars is further demonstrated by the rainbow of colors seen in a tilted optical image of a nanowire solar cell (right panel).Because of their arrangement in rows, the nanowires disperse light much like a prism.
An image taken using a scanning electron microscope (left panel) shows the effectiveness of Yang and Garnett’s growth technique. The vertically aligned pillars are each approximately 400 nanometers in diameter and five micrometers in height. The excellent periodicity of the pillars is further demonstrated by the rainbow of colors seen in a tilted optical image of a nanowire solar cell (right panel).Because of their arrangement in rows, the nanowires disperse light much like a prism.
As an added benefit of the radial p-n junction design, Yang and Garnett discovered that, due to their periodic arrangement, the silicon nanowires also trap much of the light that would usually be wasted by reflecting off the surface of the cell. Because of this enhanced absorption, thinner films of silicon can be used as the base for the nanowires—reducing the total amount of material needed and further bringing down the final cost of each cell.
Though it seems the advances made by Yang and Garnett have answered the major materials concerns with photovoltaics, much refinement is needed before nanowire solar cells will grace rooftops and provide cheap and clean energy for the everyday consumer. The best cells produced by the Berkeley researchers have an efficiency of around five percent—good for a budding laboratory technology but still far from the performance needed for a cost-effective product. For comparison, commercial planar silicon photovoltaics usually have a conversion efficiency of 15-20 percent. But if five percent seems like a meager result, consider that the first commercial silicon solar cells, developed in 1954, only achieved two percent efficiency. Nevertheless, the efficiency of the nanowire cells must be improved for commercialization to be realistic. Furthermore, the fabrication technique used to make the nanowire cells is also too costly to be viable beyond the laboratory scale. “Manufacturability is very important,” according to Garnett, “and adding one step [to the process] can be a major problem.” To address these problems, professor Yang’s research group is already looking at ways to improve device performance and make their cells cheaper to manufacture. For example, changing the size and shape of the nanowires to increase absorption and adding surface coatings to improve efficiency are potential strategies for bringing commercial nanowire solar cells to fruition. Dr. Garnett, who now works on nano-structured organic photo- voltaics at Stanford, is cautiously optimistic: “I think the benefits of silicon nanowire solar cells are pretty significant,” he says. “We hope to dramatically increase efficiency but there’s still a lot of work to be done.”
This article is part of the Spring 2010 issue.



